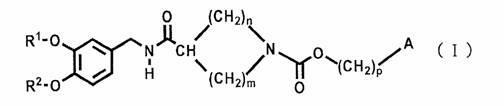
Cyclic amine-1-carboxylic acid ester derivative and pharmaceutical composition containing the same
一种甲酸酯、化合物的技术,应用在环状胺-1-甲酸酯衍生物及含有其的药物组合物领域,能够解决刺激痛强等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0240] Preparation of cis-4-ethylcyclohexyl 4-(4-hydroxy-3-methoxybenzylcarbamoyl)-piperidine-1-carboxylate:
[0241] 【Chemical 9】
[0242]
[0243] (1) In 4-benzyloxy-3-methoxybenzylamine hydrochloride (14.0g), 1-(tert-butoxycarbonyl)-piperidine-4-carboxylic acid (11.5g), triethyl To a mixture of amine (14.0ml) and dichloromethane (300ml) was added 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (12.5g). The reaction solution was stirred at room temperature for 20 hours, washed successively with saturated ammonium chloride aqueous solution and saturated brine, and the organic layer was dried over sodium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (elution solvent: hexane / ethyl acetate = gradient from 100 / 0 to 0 / 100) to obtain 4-(4-benzyloxy-3-methoxybenzylamino Formyl)-piperidine-1-carboxylic acid tert-butyl ester 16.3 g.
[0244] (2) The product (16.3 g) of the above (1) was...
Embodiment 2~12
[0250] Various substituted cyclohexanols were used instead of cis-4-ethylcyclohexanol in Example 1, and the same reaction and treatment as in Example 1 were carried out to obtain the compounds shown in Table 1 and Table 2.
[0251]
[0252]
Embodiment 13
[0254] Preparation of cis-2-ethylcyclohexyl 4-(4-hydroxy-3-methoxybenzylcarbamoyl)-piperidine-1-carboxylate:
[0255] 【chemical 10】
[0256]
[0257] Replace the cis-4-ethylcyclohexanol in Example 1 with 2-Ethylcyclohexanol, and carry out the same reaction and treatment as in Example 1. Then, the resulting cis compound was separated by silica gel column chromatography (eluting solvent: hexane / ethyl acetate = gradient from 100 / 0 to 0 / 100) to obtain the desired product.
[0258]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com