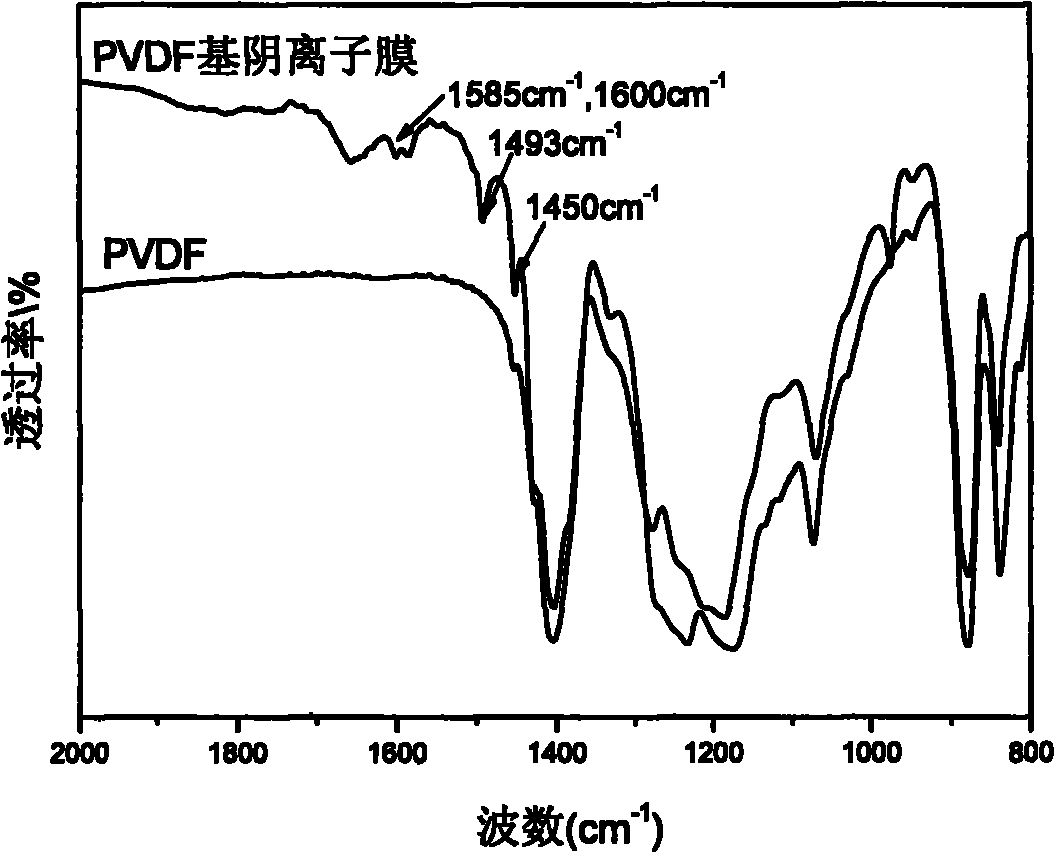
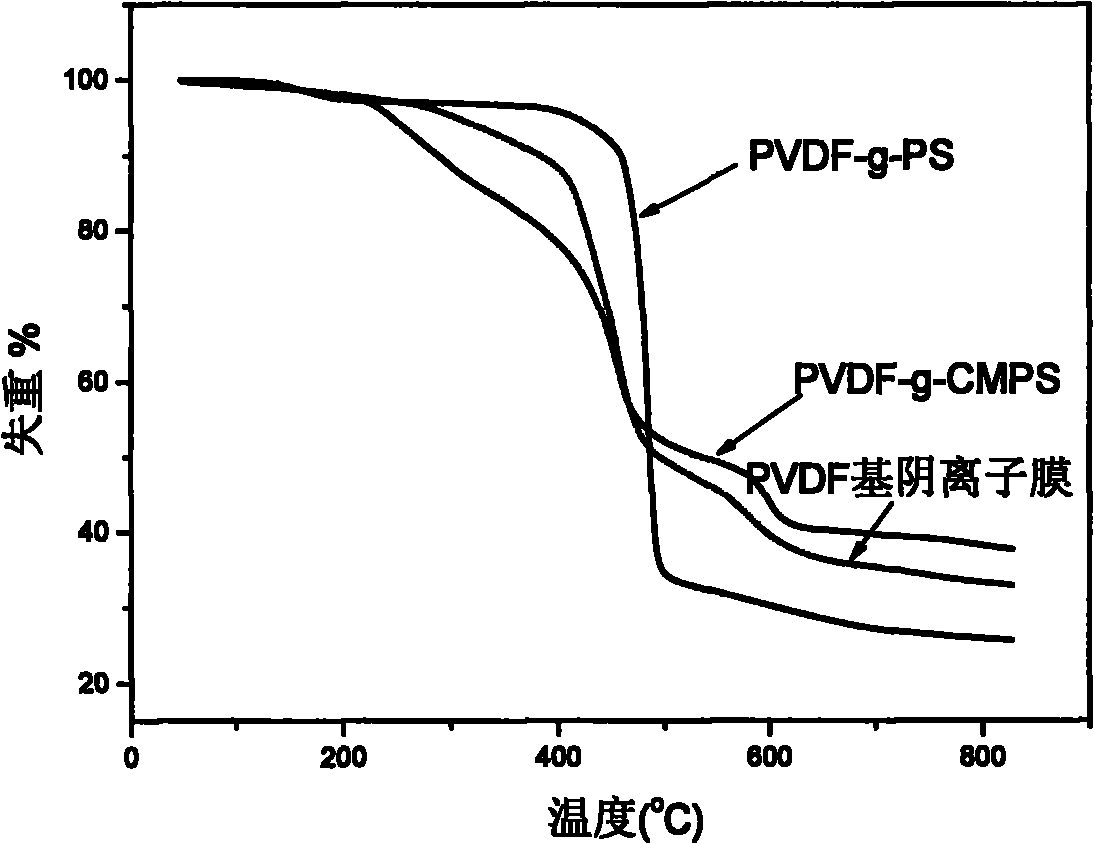
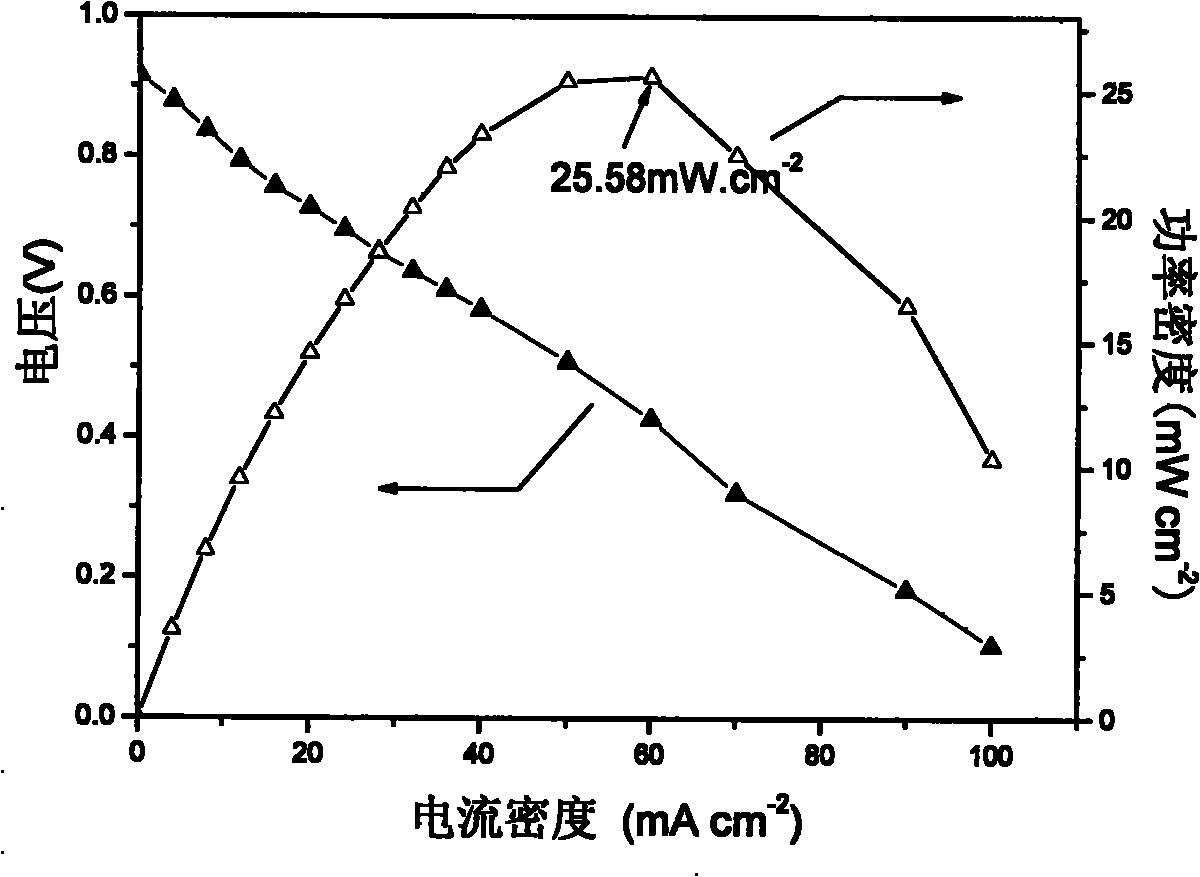
Alkaline anionic membrane and preparation method thereof
An alkaline anion and film-forming technology, applied in the field of alkaline anion membrane and its preparation, can solve the problems of poor stability and brittleness, and achieve the effects of high mechanical strength, good toughness and good battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 1 weight unit of polyvinylidene fluoride (PVDF), 0.04 weight units of cuprous chloride, and 0.154 weight units of 4,4-bipyridine into the reaction vessel, vacuumize and fill with nitrogen for 5 times, and add 20 After dissolving N-methylpyrrolidone (NMP) in units of weight, add 1 weight unit of styrene, mix for 10 minutes, and react at 120°C for 24 hours, 48 hours, and 72 hours; pour the above mixture into ethanol to make The product precipitated out, was washed repeatedly with ethanol and deionized water, and dried in vacuo. The product was dissolved in N-methylpyrrolidone (NMP) under a nitrogen atmosphere, and 0.05 weight units of tin tetrachloride and 1 weight unit of chloromethyl ethyl ether were added. React at 50°C for 2 hours. Pour the product into ethanol, filter it with suction, wash it repeatedly with toluene and deionized water, and dry it in vacuum. The next step is to dissolve the product into N,N-dimethylformamide (DMF) and form a film at 60°C by c...
Embodiment 2
[0037] Add 1 weight unit of polyvinylidene fluoride (PVDF), 0.02 weight unit of cuprous chloride, and 0.077 weight unit of 4,4-bipyridine into the reaction vessel, vacuumize and inflate nitrogen for 5 times, and add 20 After dissolving N-methylpyrrolidone (NMP) in units of weight, add 1 weight unit of styrene, mix for 10 minutes, and react at 120°C for 24 hours, 48 hours, and 72 hours; pour the above mixture into ethanol to make The product precipitated out, was washed repeatedly with ethanol and deionized water, and dried in vacuo. The product was dissolved in N-methylpyrrolidone (NMP) under a nitrogen atmosphere, and 0.05 weight units of tin tetrachloride and 1 weight unit of chloromethyl ethyl ether were added. React at 50°C for 2 hours. Pour the product into ethanol, filter it with suction, wash it repeatedly with ethanol and toluene, and dry it in vacuum. The next step is to dissolve the product into N,N-dimethylformamide (DMF) and form a film at 60°C by casting metho...
Embodiment 3
[0040]Add the polyvinylidene fluoride (PVDF) of 1 weight unit, the cuprous chloride of 0.02 weight unit, the pentamethyldiethylenetriamine (PMDETA) of 0.05 weight unit in the reaction container, vacuumize and inflate nitrogen repeatedly 5 times, use Add 20 weight units of N-methylpyrrolidone (NMP) into the syringe. After dissolving, add 1 weight unit of styrene, mix for 10 minutes, and react at 120°C for 24 hours, 48 hours, and 72 hours; pour the above mixture into The product was precipitated from ethanol, washed repeatedly with ethanol and toluene, and dried in vacuo. The product was dissolved in N-methylpyrrolidone (NMP) under a nitrogen atmosphere, and 0.05 weight units of tin tetrachloride and 1 weight unit of chloromethyl ethyl ether were added. React at 50°C for 2 hours. The product was poured into ethanol, filtered with suction, washed repeatedly with ethanol and deionized water, and dried in vacuum. The next step is to dissolve the product into N,N-dimethylformami...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com