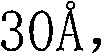Gas phase purification method for 1,1-difluoroethane
A technology of difluoroethane and purification method, which is applied in 1 field and can solve problems such as not being able to meet high purity requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
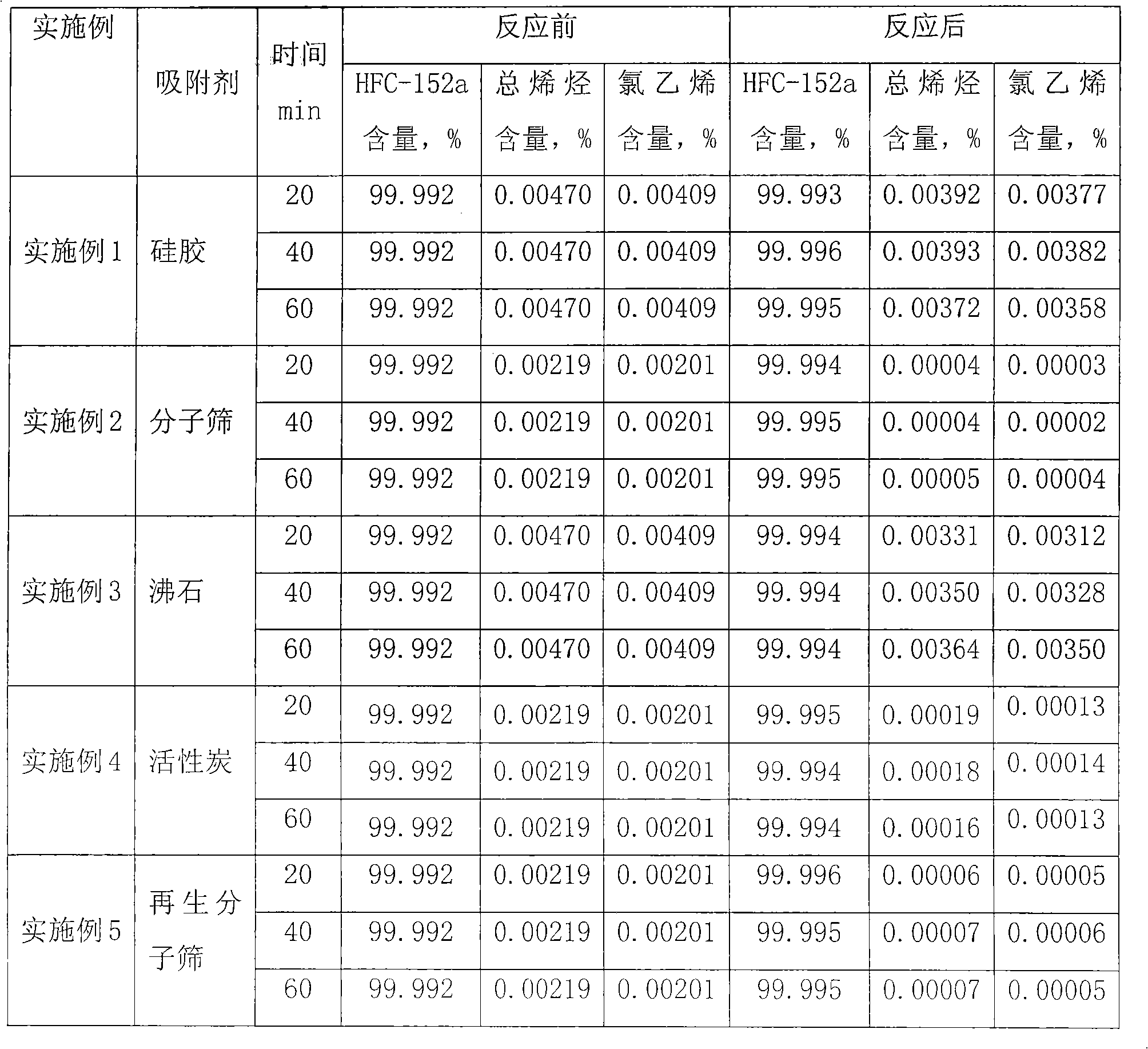
[0028] Add 17.5g (33ml) of silica gel to the reaction tube, specific surface area: 750-850m 2 / g, average pore diameter: 22- Heating keeps the temperature in the reaction tube at about 50°C and the pressure at about 2 atmospheres, feeds chlorine gas into the reaction tube at a flow rate of 0.0) L / min for 2 hours, closes the valve of the reaction tube, and seals it for 2 hours. Heating was stopped, the valve was opened, the reaction tube was purged with nitrogen, vacuumized, and the reaction tube was naturally cooled to normal temperature. The crude product of 1,1-difluoroethane containing vinyl chloride was passed into the reaction tube at a speed of 0.1L / min. The content of 1,1-difluoroethane was 99.992%, and the content of vinyl chloride was 0.00201%. The olefin content was 0.00219%, and the pressure during the adsorption process was kept at about 5 atmospheres. Samples were taken after 20, 40 and 60 minutes for gas chromatography analysis. The results are shown in Table 1...
Embodiment 2
[0030] Add 140g (265ml) molecular sieve into the reaction tube, the specific surface area is 830-860m 2 / g, average pore diameter: 14- Heating to keep the temperature in the reaction tube at about 350°C, and the pressure at about 5 atmospheres, feed chlorine gas into the reaction tube at a flow rate of 0.05 L / min for 3 hours, close the valve of the reaction tube, and seal it for 2 hours. Heating was stopped, the valve was opened, the reaction tube was purged with nitrogen, vacuumized, and the reaction tube was naturally cooled to normal temperature. The crude 1,1-difluoroethane containing vinyl chloride is introduced into the reaction tube at a speed of 0.1 L / min. The content of 1,1-difluoroethane is 99.992%, the content of vinyl chloride is 0.00201%, and the total olefins The content is 0.00219%, and the pressure in the adsorption process is maintained at about 3 atmospheres. Samples are taken after 20, 40 and 60 minutes for gas chromatography analysis, and the results are ...
Embodiment 3
[0032] Add 17.5g (33ml) zeolite to the reaction tube, the specific surface area is 620-750m 2 / g, average pore diameter 22- Heating to keep the temperature in the reaction tube at about 350°C, and the pressure at about 8 atmospheres, feed chlorine gas into the reaction tube at a flow rate of 0.05 L / min for 10 hours, close the valve of the reaction tube, and seal it for 2 hours. Heating was stopped, the valve was opened, the reaction tube was purged with nitrogen, vacuumized, and the reaction tube was naturally cooled to normal temperature. The crude product of 1,1-difluoroethane containing vinyl chloride was passed into the reaction tube at a speed of 0.1L / min. The content of 1,1-difluoroethane was 99.992%, and the content of vinyl chloride was 0.00201%. The olefin content was 0.00219%, and the pressure during the adsorption process was maintained at about 8 atmospheres. Samples were taken after 20, 40 and 60 minutes for gas chromatography analysis. The results are shown in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com