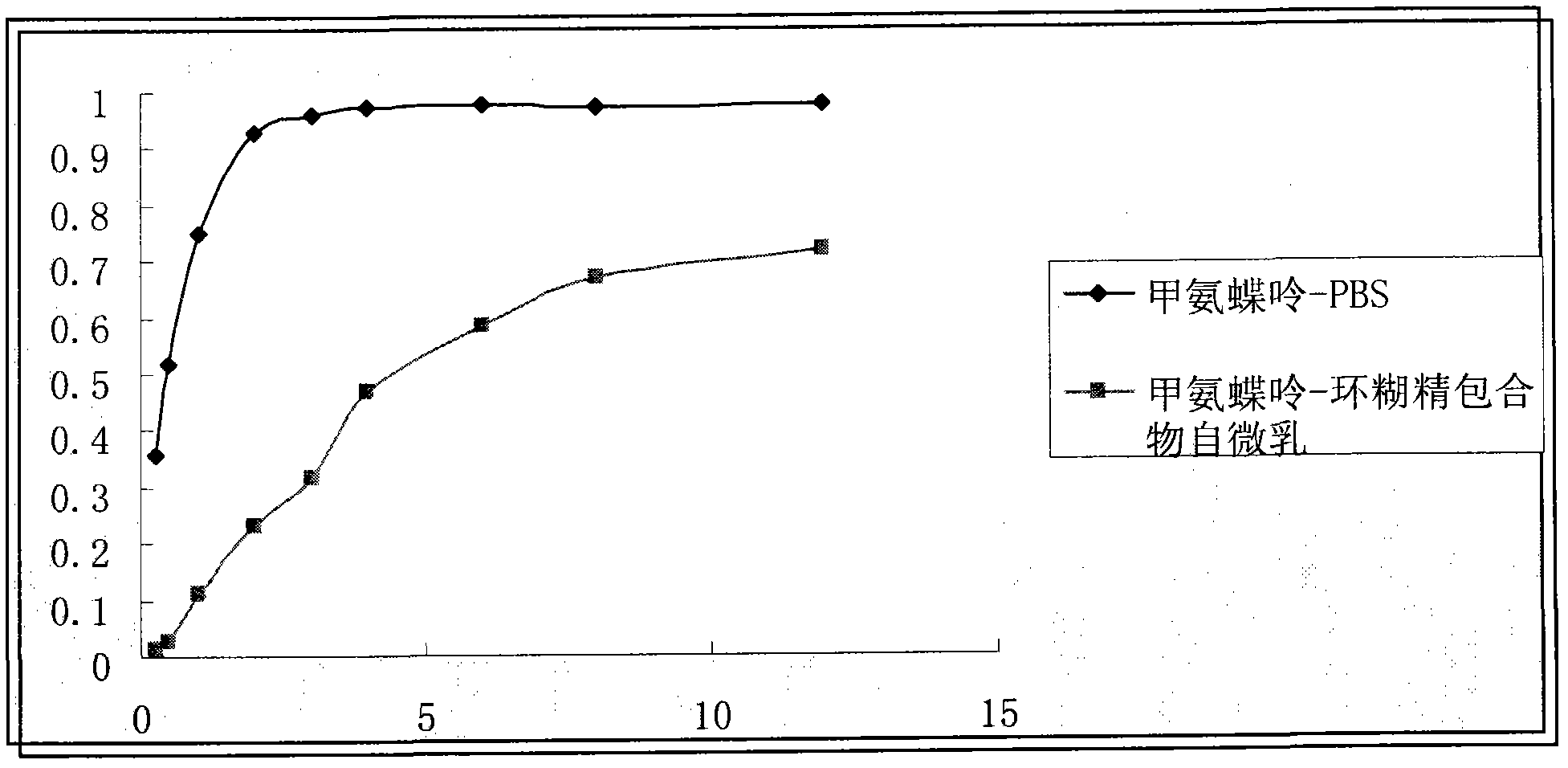
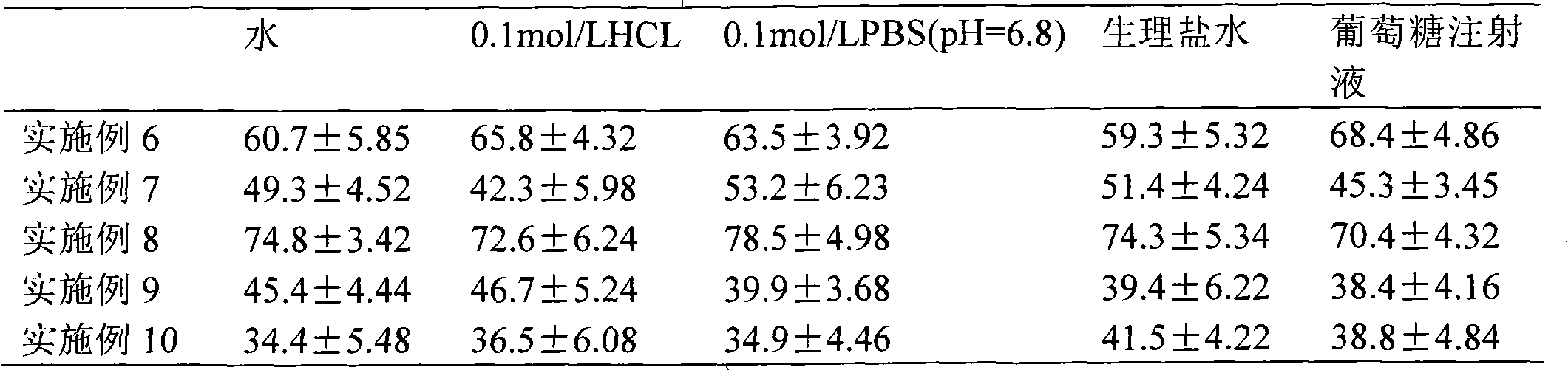
Preparation method and application of medicament-cyclodextrin inclusion compound self-emulsifying composition
A technology of cyclodextrin inclusion complex and composition, which is applied in directions such as pharmaceutical formulations, medical preparations of inactive ingredients, and emulsion delivery, can solve the problems of difficult preparation of preparations, low bioavailability and the like, and achieves a simple preparation process. Ease of operation, good emulsifying performance, and low cost of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation and content determination of methotrexate-β-cyclodextrin inclusion compound
[0040] Weigh 7mmol β-cyclodextrin and 1mmol methotrexate in a mortar, add an appropriate amount of ammonia water containing 35% NH4OH, grind for 30min, dry in a vacuum oven for 12h to obtain a solid powder, add appropriate amount of water, and centrifuge to remove the precipitate ( free drug), the supernatant was taken, and vacuum-dried to obtain the methotrexate-cyclodextrin inclusion compound.
[0041] Methotrexate content was determined by HPLC (LC-2010C, Shimadzu, Japan) method. The mobile phase is methanol: water=75:25 (v / v), and the chromatographic column is Lichrospher C 18 (150×4.6μm), the column particle size is 5μm. The flow rate was 1.0 mL / min, the detection wavelength was 227 nm (SPD-10A, UV detector, Shimadzu, Japan), the column temperature was 30° C., and the injected sample volume was 20 μl. The drug loading amount of the sample was calculated by formul...
Embodiment 2
[0043] Example 2: Preparation and Content Determination of Retinoic Acid-β-Cyclodextrin Inclusion Compound
[0044] Weigh 10mmol β-cyclodextrin and 1mmol retinoic acid in a mortar, add an appropriate amount of ammonia water containing 35% NH4OH, grind for 30min, dry in a vacuum oven for 12h to obtain a solid powder, add appropriate amount of water, and centrifuge to remove the precipitate (free drug ), take the supernatant, and vacuum-dry to obtain retinoic acid-cyclodextrin inclusion compound.
[0045]The content determination was carried out by HPLC (LC-2010C, Shimadzu, Japan) method. The mobile phase is methanol: water: formic acid=95:5:0.5 (v / v), and the chromatographic column is Lichrospher C 18 (150×4.6μm), the column particle size is 5μm. The flow rate was 1.0 mL / min, the detection wavelength was 345 nm (SPD-10A, UV detector, Shimadzu, Japan), the column temperature was 25° C., and the injected sample volume was 20 μl. The encapsulation efficiency of the sample was c...
Embodiment 3
[0046] Example 3: Preparation and content determination of simvastatin-hydroxypropyl-β-cyclodextrin inclusion compound
[0047] Weigh 5mmol hydroxypropyl-β-cyclodextrin and 1mmol rosuvastatin, add 10ml water, stir magnetically at 37°C for 72h, after reaching stability, centrifuge the sample, pass the supernatant through the membrane (to remove free drug), freeze dry to obtain simvastatin-cyclodextrin inclusion complex.
[0048] The content determination was carried out by HPLC (LC-2010C, Shimadzu, Japan) method. The mobile phase is 0.025mol L -1 Sodium dihydrogen phosphate solution (p H=4.5)-acetonitrile (35:65) (v / v), the chromatographic column is Lichrospher C 18 (150×4.6 μm). The flow rate was 1.0 mL / min, the detection wavelength was 238 nm (SPD-10A, UV detector, Shimadzu, Japan), the column temperature was 40°C, and the injected sample volume was 20 μl. The encapsulation efficiency of the sample was calculated by formula (1). As a result of calculation, the encapsulat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com