Preparation method of fasudil hydrochloride
A technology of fasudil hydrochloride and amine group, applied in the field of preparation of pharmaceutical compounds, can solve the problems of difficulty in separation, high commercial price, affecting the production cost of final products, etc., and achieve the effects of improving yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
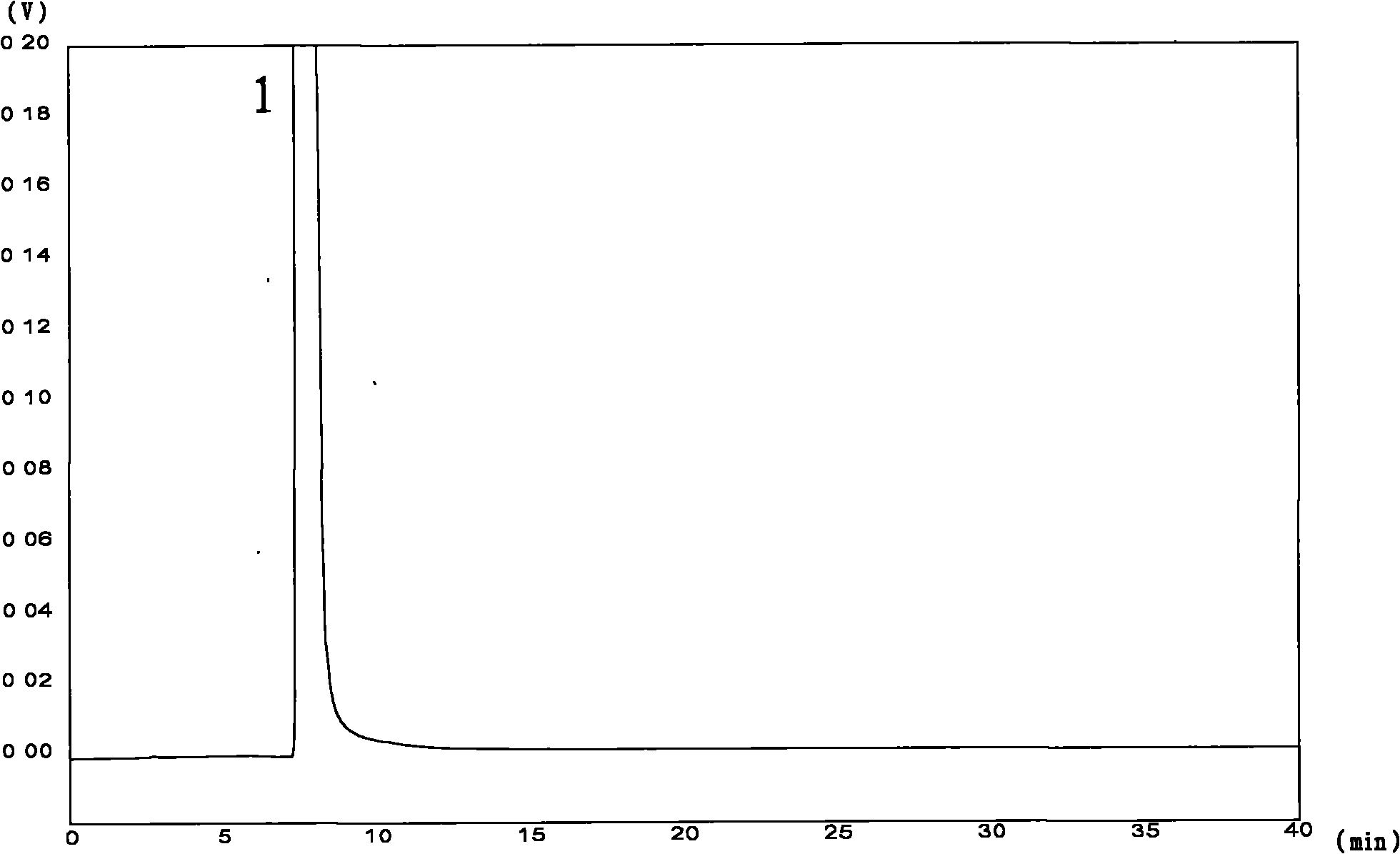
Image
Examples
Embodiment 1
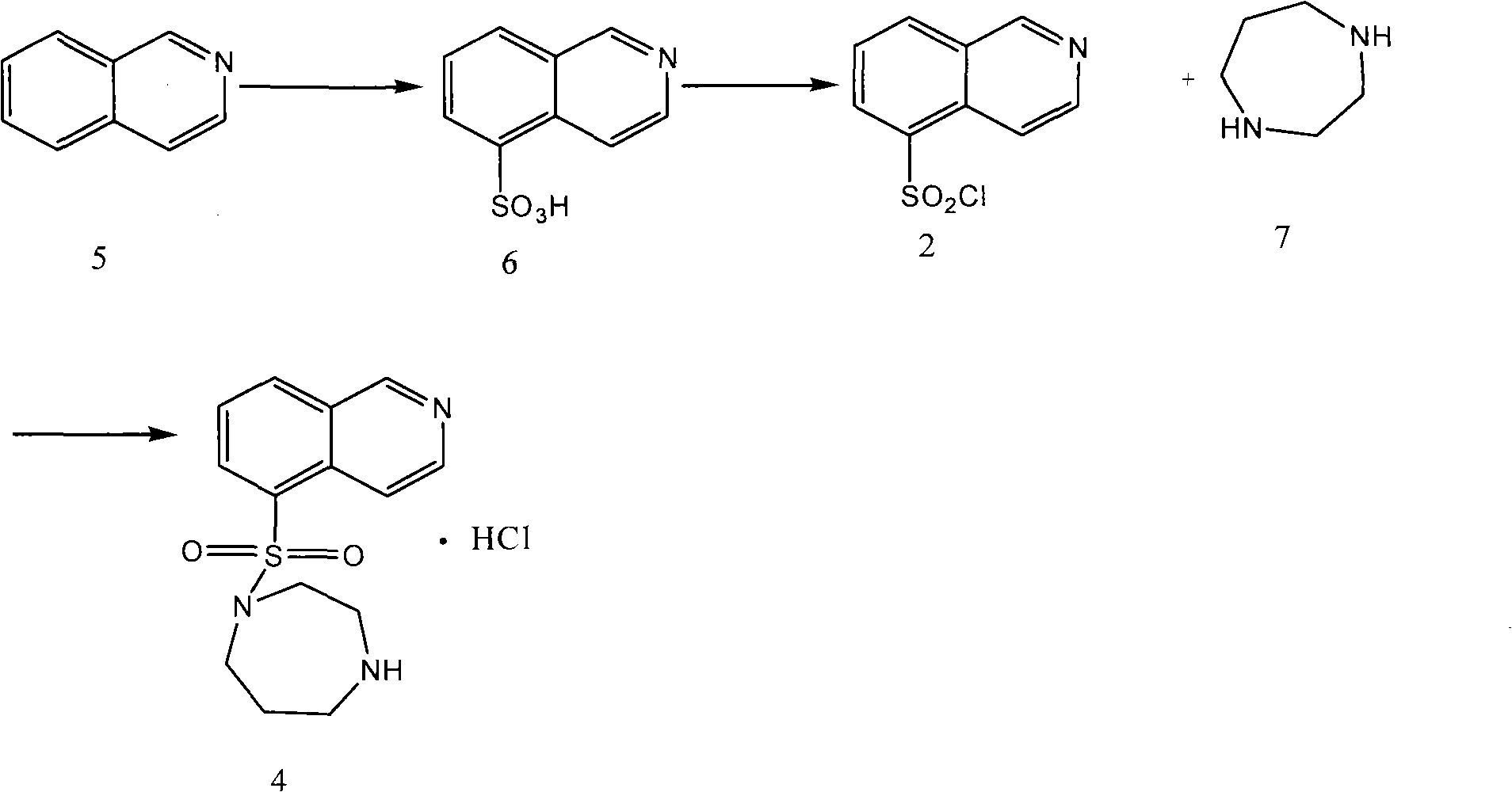
[0032] Embodiment 1 prepares 1-benzyloxycarbonyl-4-(5-isoquinoline sulfonamide) homopiperazine
[0033] Add 1-benzyloxycarbonylhomopiperazine (1.23 kg), 5-isoquinolinesulfonyl chloride (1 kg) and potassium carbonate (0.88 kg), add dichloromethane (5 liters), and stir vigorously at 15-30°C 5 hours. After completion of the reaction, add water (5 liters), separate the layers, extract the aqueous phase with ethyl acetate (5 liters each time, extract 3 times), combine the organic layers, dry with anhydrous sodium sulfate for 3 hours, and filter off the solids. The filtrate was concentrated under reduced pressure to obtain 1-benzyloxycarbonyl-4-(5-isoquinolinesulfonamide)homopiperazine as a pale yellow solid. It can be used directly without purification.
Embodiment 2
[0034] Example 2 Preparation of Hexahydro-1-(5-isoquinolinesulfonyl)-1H-1,4-diazepine hydrochloride
[0035] Using the 1-benzyloxycarbonyl-4-(5-isoquinolinesulfonamide) homopiperazine (1 kg) prepared as shown in Example 1, add 10 liters of water, carefully add concentrated hydrochloric acid (10 liter), after the exotherm stabilizes, heat to 100-110°C for reflux reaction for 3 hours. After the reaction was completed, the reaction solution was naturally cooled to room temperature, extracted with ethyl acetate (2 liters each time, extracted 3 times in total), collected the aqueous phase, adjusted to pH=10-13 with 5M sodium hydroxide solution, and extracted with dichloromethane (3 liters each time, total extraction 4 times), the organic phases were combined, dried with anhydrous sodium sulfate for 3 hours, the solid was filtered off, and the filtrate was concentrated under reduced pressure to obtain a light yellow solid and the crude product of Fasudil. The obtained pale yellow s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com