Use of butyl phthalide and derivatives thereof in preparation of medicines for treating Parkinson disease
A technology for Parkinson's disease and derivatives, which is applied to the application field of butylphthalide and its derivatives in the preparation of medicines for treating Parkinson's disease, and can solve the problem of not teaching butylphthalide and its derivatives Parkinson's disease medicines, etc. problem, to achieve the effect of exact curative effect, improvement of pathogenesis, prevention or delay of progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 Butylphthalide
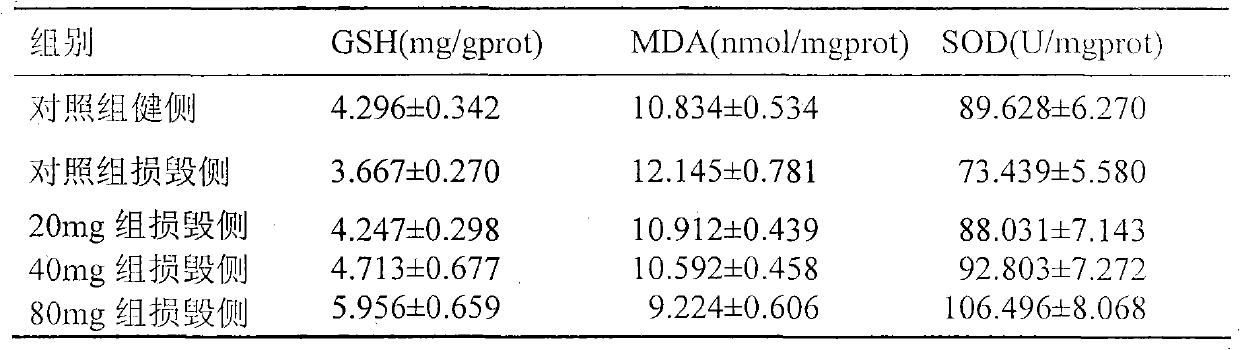
[0012] Observation in the rotenone PD rat model: intraperitoneal injection of butylphthalide to PD rats, and observe the effects of butylphthalide on the behavior, midbrain oxidative stress level and substantia nigra DA neurons of PD rats.
[0013] 1. Experimental materials
[0014] 1. Drug: butylphthalide, provided by CSPC NBP Pharmaceutical Co., Ltd.
[0015] 2. Reagents: Rotenone and apomorphine (APO) are products of Sigma; dimethyl sulfoxide (DMSO), tyrosine hydroxylase (TH) monoclonal antibody, and SABC kit are products of Wuhan Boster; GSH reagents The kit, MDA kit and SOD kit are products of Nanjing Jiancheng Company.
[0016] 3. Instruments: stereotaxic instrument for rat brain, Shenzhen Ruiwode Company; micro sample injector (5μl), Shanghai Third Analytical Instrument Factory; 721 spectrophotometer, Shanghai Precision Scientific Instrument Co., Ltd.; optical microscope, NikonYS100.
[0017] 4. Experimental animals: 50 female SD rat...
Embodiment 2
[0042] Example 2 L-Butylphthalide
[0043] Observation in the rotenone PD rat model: intraperitoneal injection of L-butylphthalide to PD rats, and observe the effects of L-butylphthalide on the behavior, midbrain oxidative stress level and substantia nigra DA neurons of PD rats.
[0044] One, test material is the same as embodiment 1
[0045] Two, test method is the same as embodiment 1
[0046] 3. Experimental results
[0047] (1) Observation of rotation behavior
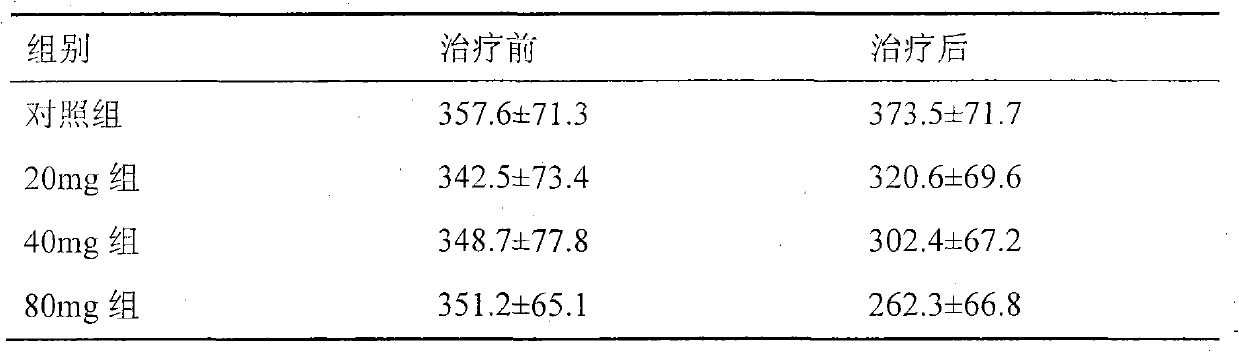
[0048] 1. There was no significant difference in rotation behavior between the control group and each treatment group before treatment (P>0.05).
[0049] 2. Compared with before treatment, the number of rotations in the control group increased after treatment, and the number of rotations in the 20mg group decreased, but there was no statistical significance (P>0.05). The number of rotations in the 40mg and 80mg groups decreased after treatment compared with before treatment, and there was a significant differen...
Embodiment 3
[0071] Example 3 Propylenephthalide
[0072] Observation in the rotenone PD rat model: intraperitoneal injection of propylphthalide to PD rats to observe the effects of propylphthalide on the behavior, midbrain oxidative stress level and substantia nigra DA neurons of PD rats.
[0073] One, test material is the same as embodiment 1
[0074] Two, test method is the same as embodiment 1
[0075] 3. Experimental results
[0076] (1) Observation of rotation behavior
[0077] 1. There was no significant difference in rotation behavior between the control group and each treatment group before treatment (P>0.05).
[0078] 2. Compared with before treatment, the number of rotations in the control group increased after treatment, and the number of rotations in the 20mg and 40mg groups decreased, but there was no statistical significance (P>0.05). The number of rotations in the 80mg group decreased after treatment compared with that before treatment, and there was a significant diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com