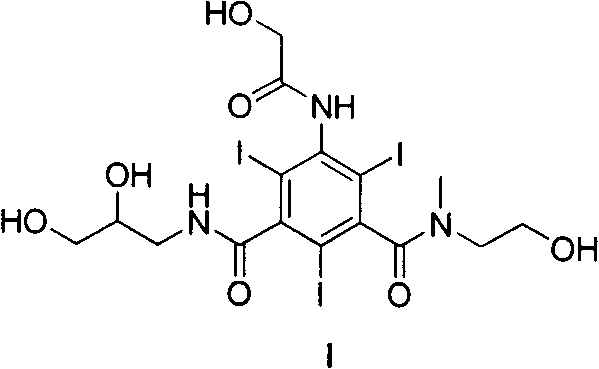
Triiodobenzene compound and contrast medium with same
A technology of compound and contrast agent, which is applied in the field of 5--N-methyl-N--N'--2, triiodobenzene compound and the contrast agent containing the compound, which can solve the problem of toxicity, hyperosmosis, high viscosity, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
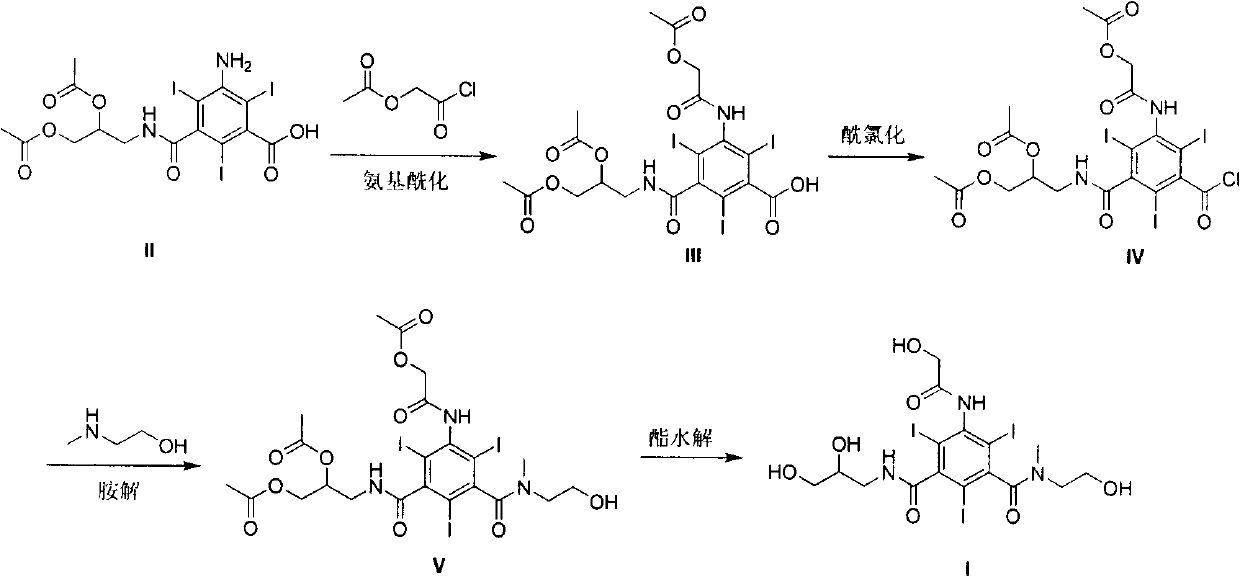
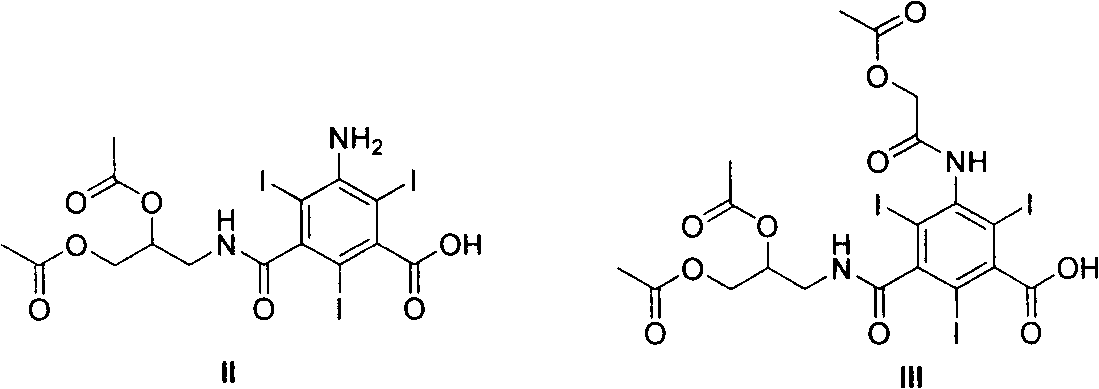
[0030] Example 1 3-Acetoxyacetamido-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (III)
[0031]
[0032] Under ice-water bath cooling and mechanical stirring, to 3-amino-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (II, 150g, 0.21mol) Acetoxyacetyl chloride (68.2 g, 0.50 mol) was added dropwise to the tetrahydrofuran (450 ml) solution, and the drop was completed (4 h), then stirred at room temperature, followed by TLC, and the reaction was completed after 28 h. Concentration under reduced pressure gave viscous material III (136.8 g, 80%). The next reaction (acyl chloride) was carried out without purification. 1 HNMR (CDCl 3 , 400MHz): δ: 2.01(s, 3H), 2.07(s, 3H), 2.13(s, 3H), 3.06-4.27(m, 4H), 4.93(s, 2H), 5.11(m, 1H), 8.51 (brs, 1H), 9.95-10.05 (m, 1H), 12.81 (brs, 1H); MS (ESI, m / z): 817 (M+H) + .
Embodiment 2
[0033] Example 2 3-Acetoxyacetamido-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoyl chloride (IV)
[0034]
[0035] 3-Acetoxyacetamido-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (III, 136.8g, 0.17mol) obtained in Example 1 , a mixture of chloroform (400ml) and triethylamine (6.8ml) was heated to reflux, and a solution of bis(trichloromethyl)carbonate (32.7g, 0.11mol) in chloroform (100ml) was added dropwise under stirring, and the dropwise completion (6h) , Stir the reaction at the same temperature for 8h. After concentration under reduced pressure, the resulting residue was recrystallized from THF (300ml) to obtain IV as an off-white solid (102.1g, 73%). 1 HNMR (CDCl 3 , 400MHz): δ2.05(s, 3H), 2.11(s, 3H), 2.17(s, 3H), 3.09-4.23(m, 4H), 4.96(s, 2H), 5.23(m, 1H), 8.58 (brs, 1H), 10.05-10.25 (brs, 1H); MS (ESI, m / z): 835 (M+H) + .
Embodiment 3
[0036] Example 3 5-Acetoxyacetamido-N-methyl-N-(2-hydroxyethyl)-N'-(2,3-diacetoxy-n-propyl)-2,4,6- Triiodoisophthalamide (V)
[0037]
[0038] The obtained 3-acetoxyacetamido-5-(2,3-diacetoxy-n-propylcarbamoyl)-2,4,6-triiodobenzoyl chloride (IV, 174.8g, 0.21mol ), chloroform (700ml) and anhydrous sodium carbonate (6g, 0.059mol) were cooled to -5°C with an ice-salt bath, nitrogen methyl ethanolamine (19.7g, 0.26mol) was added dropwise under stirring, and the dropwise (3.5h ). The ice-salt bath was removed, and the reaction was stirred at room temperature for 12h. The reaction mixture was then cooled to -5°C with an ice-salt bath, and nitrogenmethylethanolamine (19.7 g, 0.26 mol) was added dropwise within 3.5 h with stirring. The ice-salt bath was removed, the reaction was stirred at room temperature, and the reaction progress was tracked by TLC, and the reaction was completed within 26 hours. The insoluble matter was filtered off, and the filtrate was concentrated under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com