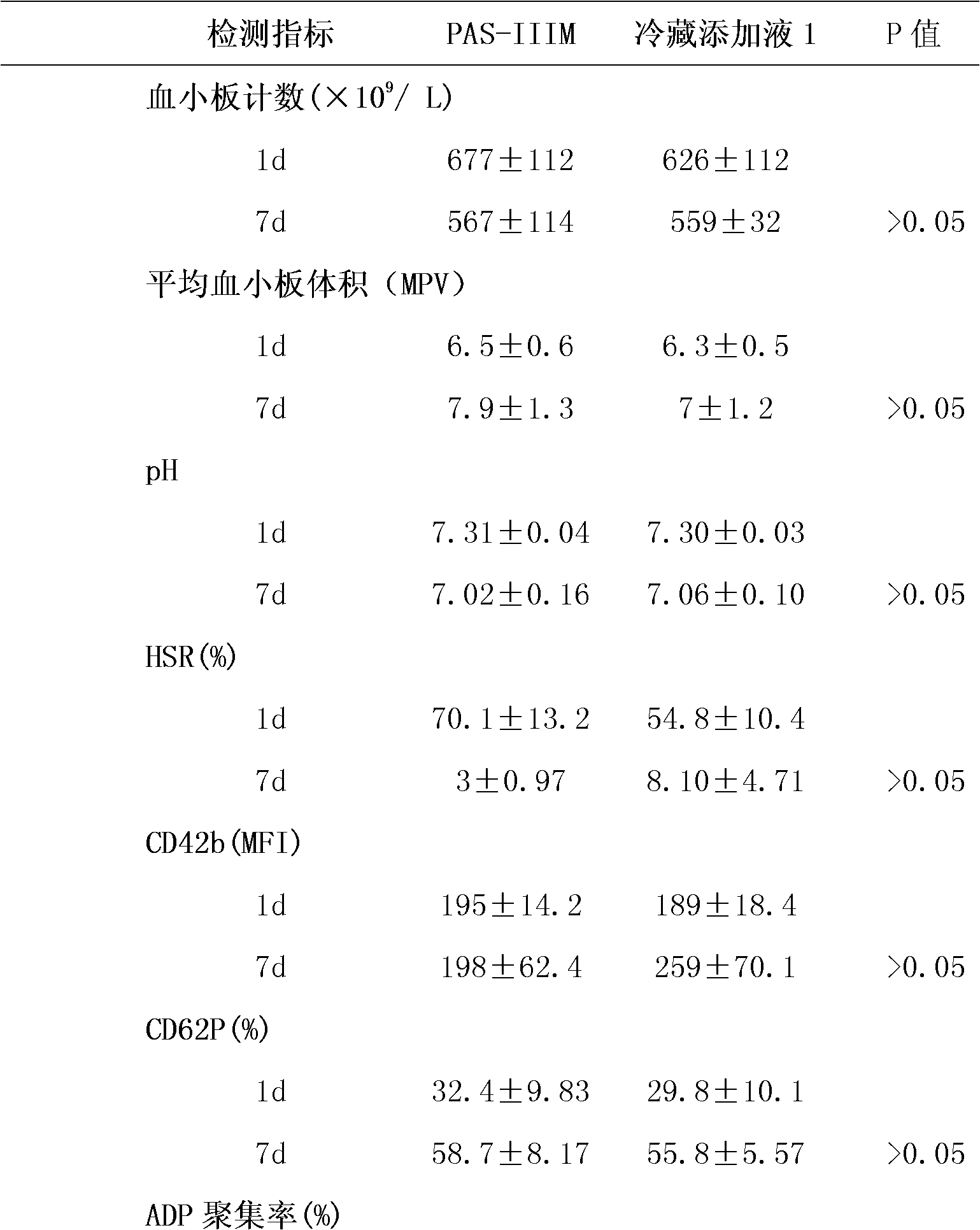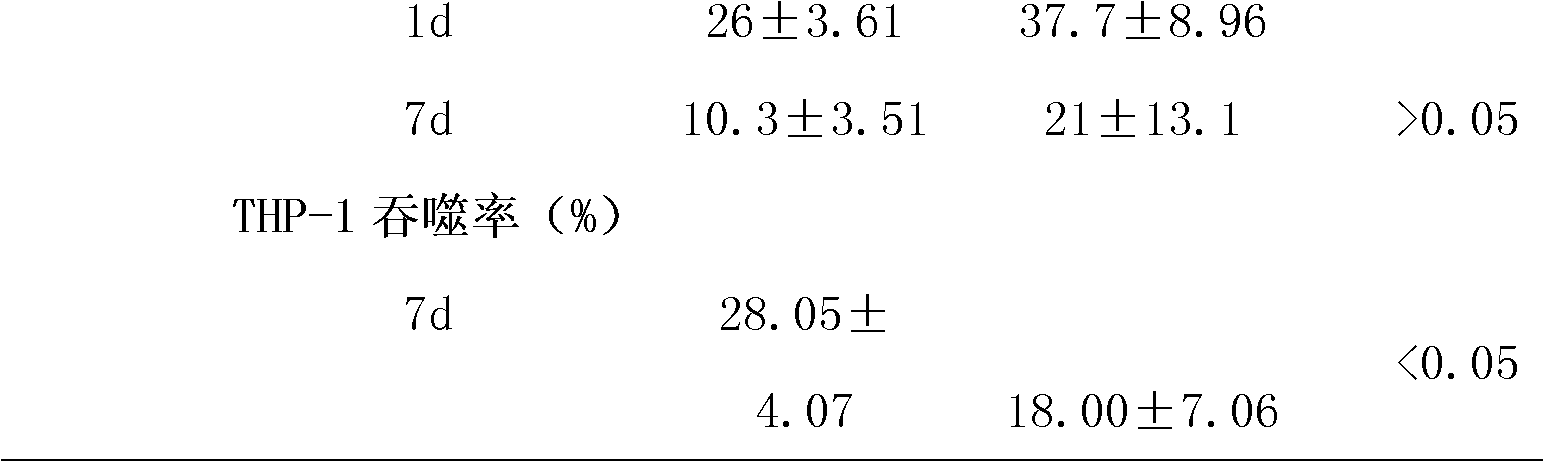Adding liquid for storing platelets under liquid low temperature condition and application thereof
A technology of platelets and additive solution, which is applied in the fields of application, preservation of human or animal body, animal husbandry, etc., can solve the problems such as the advent of platelet refrigeration additive solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prepare platelet additive solution according to the formula in Table 1.
[0036] The preparation method is as follows: taking blood anticoagulant, platelet metabolism substrate, balance salt, and cold storage protection agent according to the formula, adding ion water, fully stirring and dissolving, and then filter sterilization or high-pressure steam sterilization.
[0037] Table 1 (unit mmol / L)
[0038] composition
Embodiment 2
[0040] Under aseptic conditions, each unit of apheresis platelet sample was divided into two parts, which were placed in special platelet storage bags, centrifuged (1000xg, 15min), and 35% of plasma was retained, and the refrigerated supplement solution 1 ( Contains trehalose and mannitol, the formula is shown in Table 2) and PAS-IIIM (SSP+, Macopharma Inc. has been clinically used for platelet storage at 22°C in Europe) platelet supplement solution, and stored at 4°C for 7 days.
[0041] Table 2.
[0042] composition
Concentration (mmoL / L)
69
[0043] sodium citrate
10
26
26
5
1.5
sodium acetate
30
50
80
[0044] 1. Platelets were stored for 7 days, and samples were taken, and the platelet count and average platelet volume...
Embodiment 3
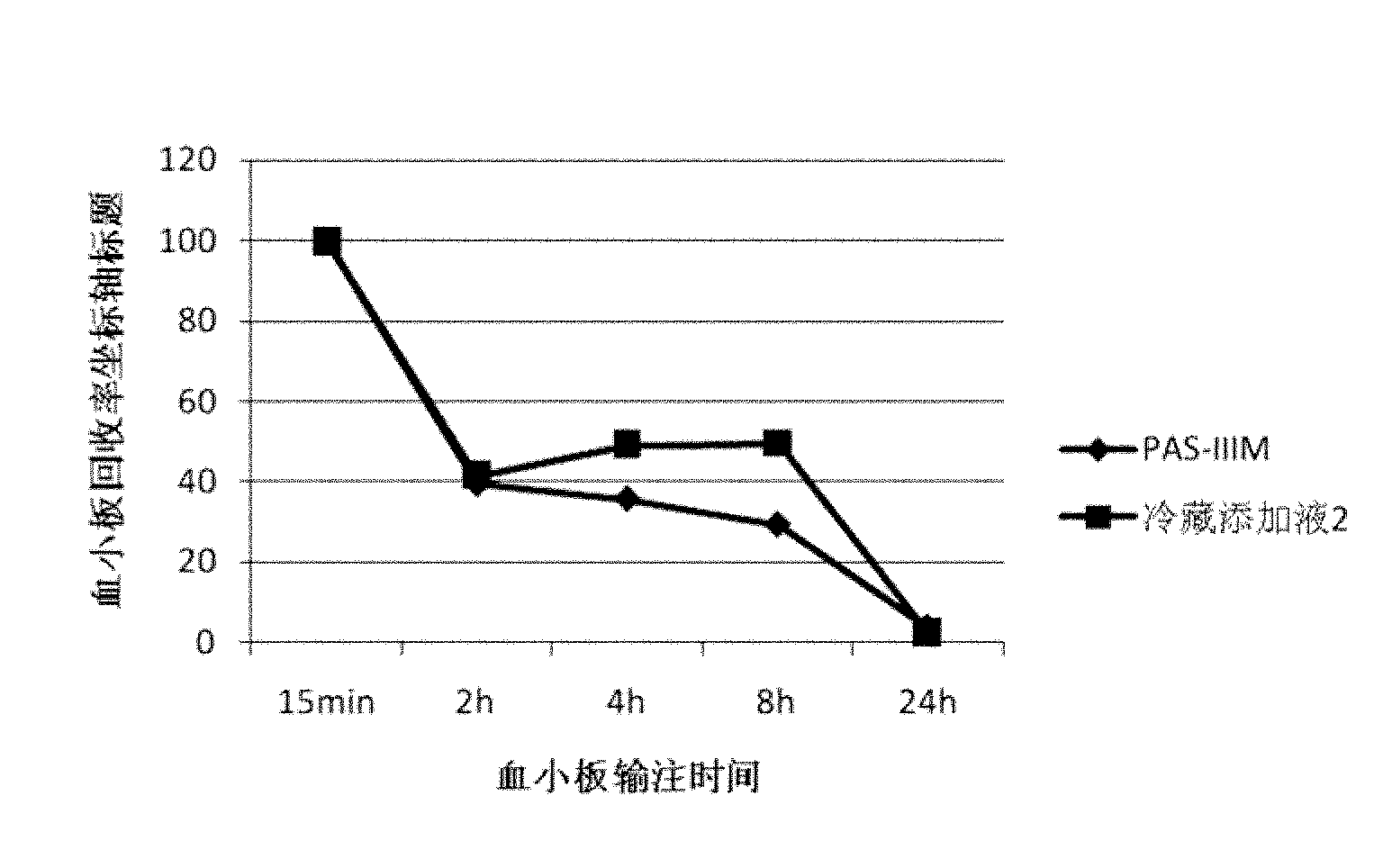
[0051] Under aseptic conditions, each unit of apheresis platelet sample was divided into two parts, which were placed in special platelet storage bags, centrifuged (1000xg, 15min), and 35% of plasma was retained, and the refrigerated supplement solution 2 ( Contains DMSO and mannitol, the formula is shown in Table 4) and PAS-IIIM (SSP+, Macopharma Inc. has been clinically used for platelet preservation at 22°C in Europe) platelet supplement solution, and stored at 4°C for 7 days.
[0052] Table 4.
[0053] composition
Prescription amount (mmoL / L)
Sodium chloride
69
sodium citrate
10
26
26
potassium chloride
5
magnesium chloride
1.5
30
DMSO
5%
80
[0054] Platelets were stored for 7 days and samples were taken, and the platelet count and mean platelet volume were measured with a pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com