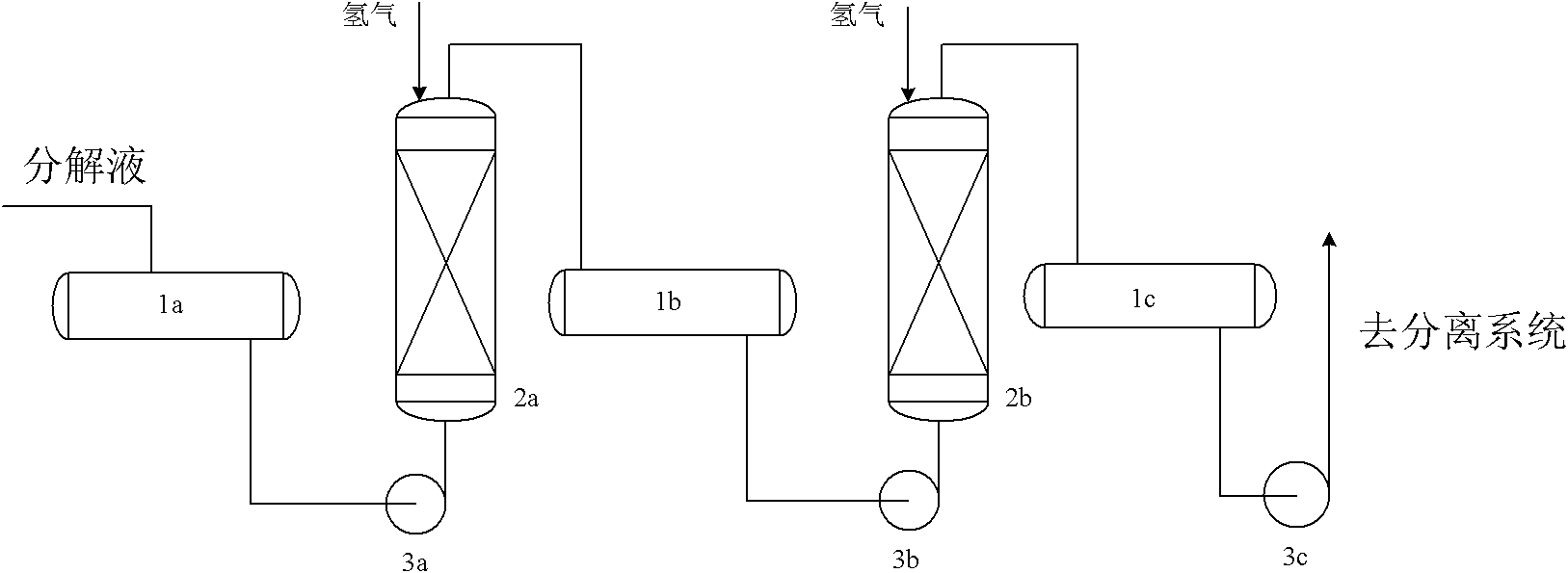
Method for treating products of alkylbenzene hydroperoxide decomposition reaction
A hydrogen peroxide and alkylbenzene technology, applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carbonyl compounds, etc., to achieve the effect of increasing yield and reducing hydrogenation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
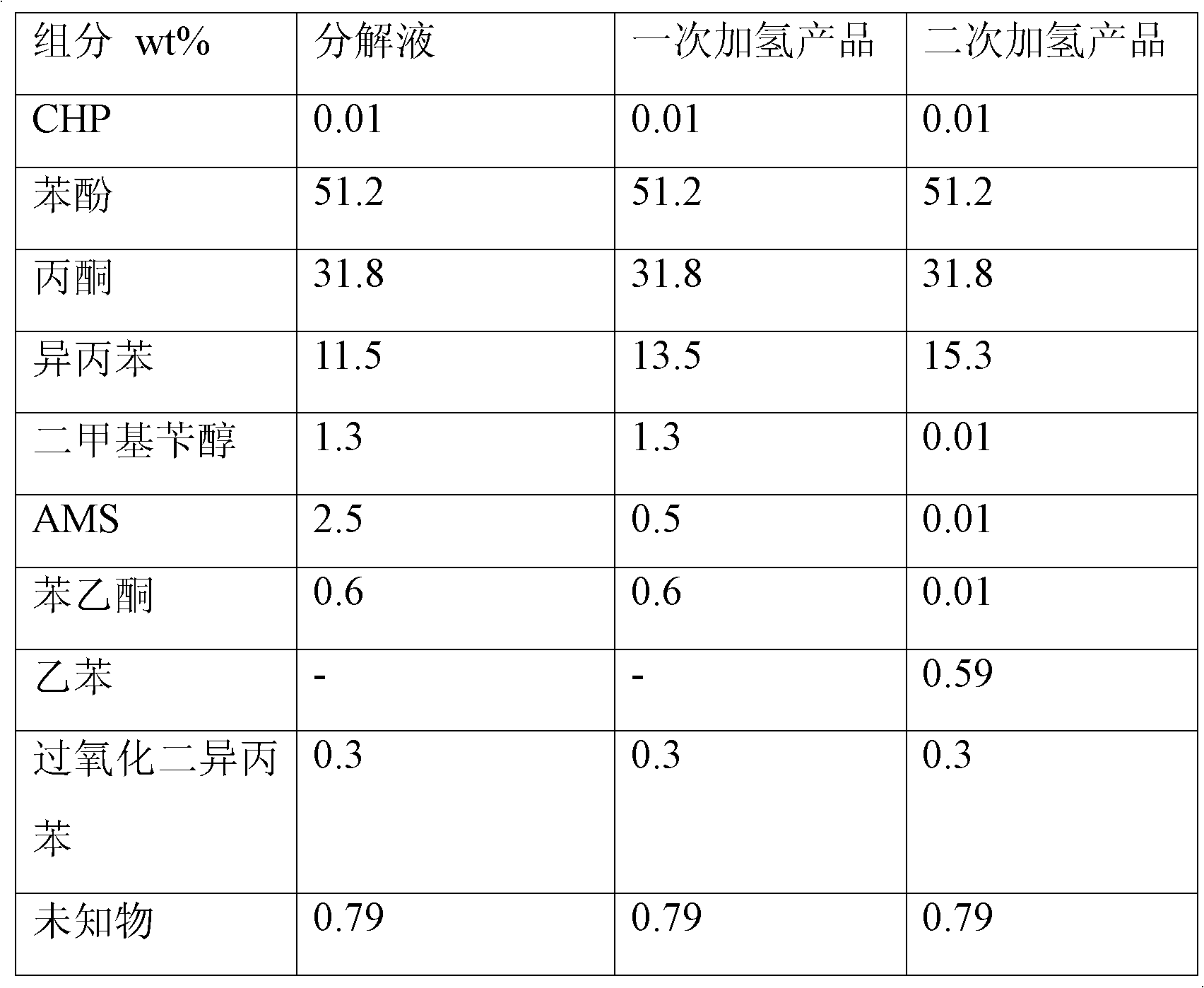
[0014] The decomposition solution containing the composition shown in Table 1 is injected into the first hydrogenation reactor, wherein nickel-cerium-Al is filled 2 o 3 Catalyst, at 60 ℃, under normal pressure condition, carry out hydrogenation treatment, obtain the content of product AMS and drop to 0.5%, then enter the second hydrogenation reactor, wherein packing palladium carbon catalyst (palladium content is 10wt%), temperature is 200 ℃, the hydrogen pressure rises to 0.5MPa, dimethyl benzyl alcohol and the remaining AMS are converted into cumene, and acetophenone is converted into ethylbenzene. During the hydrogenation process, hydrogenation of the benzene ring cannot occur, and the product mainly contains phenol , acetone, cumene and ethylbenzene, as well as high-boiling impurities, easy to separate.
[0015] Table 1:
[0016]
Embodiment 2
[0018] The decomposition solution containing the composition shown in Table 2 is injected into the first hydrogenation reactor, wherein nickel-cerium-Al is filled 2 o 3 Catalyst, at 70 ℃, carry out hydrogenation treatment under normal pressure condition, obtain the content of product AMS and drop to 0.4%, then enter the second hydrogenation reactor, wherein packing palladium carbon catalyst (palladium content is 10wt%), temperature is 200 ℃, the hydrogen pressure rises to 1.0MPa, dimethyl benzyl alcohol and the remaining AMS are converted into cumene, and acetophenone is converted into ethylbenzene. During the hydrogenation process, hydrogenation of the benzene ring cannot occur, and the product mainly contains phenol , acetone, cumene and ethylbenzene, as well as high-boiling impurities, easy to separate.
[0019] Table 2
[0020]
Embodiment 3
[0022] The decomposition solution containing the composition shown in Table 3 is injected into the first hydrogenation reactor, wherein nickel-cerium-Al is filled 2 o 3 Catalyst, at 70 ℃, under normal pressure condition, carry out hydrogenation treatment, obtain the content of product AES and drop to 0.5%, then enter the second hydrogenation reactor, wherein packing palladium carbon catalyst (palladium content is 10wt%), temperature is 200 ℃, the hydrogen pressure rises to 1.0MPa, methyl ethyl benzyl alcohol and the remaining AES are converted into sec-butylbenzene, and acetophenone is converted into ethylbenzene. During the hydrogenation process, hydrogenation of the benzene ring cannot occur, and the main product is Contains phenol, methyl ethyl ketone, sec-butylbenzene and ethylbenzene, as well as high boiling impurities, easy to separate.
[0023] table 3
[0024] Component wt%
[0025] Acetophenone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com