Application of alpha-mannatide as vaccine adjuvant and vaccine preparation prepared using same
A technology of mannan peptides and vaccine adjuvants, which is applied to medical preparations containing active ingredients, antiviral agents, antibody medical components, etc., can solve problems such as no immune response, reduce the amount of antigen used, and induce cellular immunity Response, immune response enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
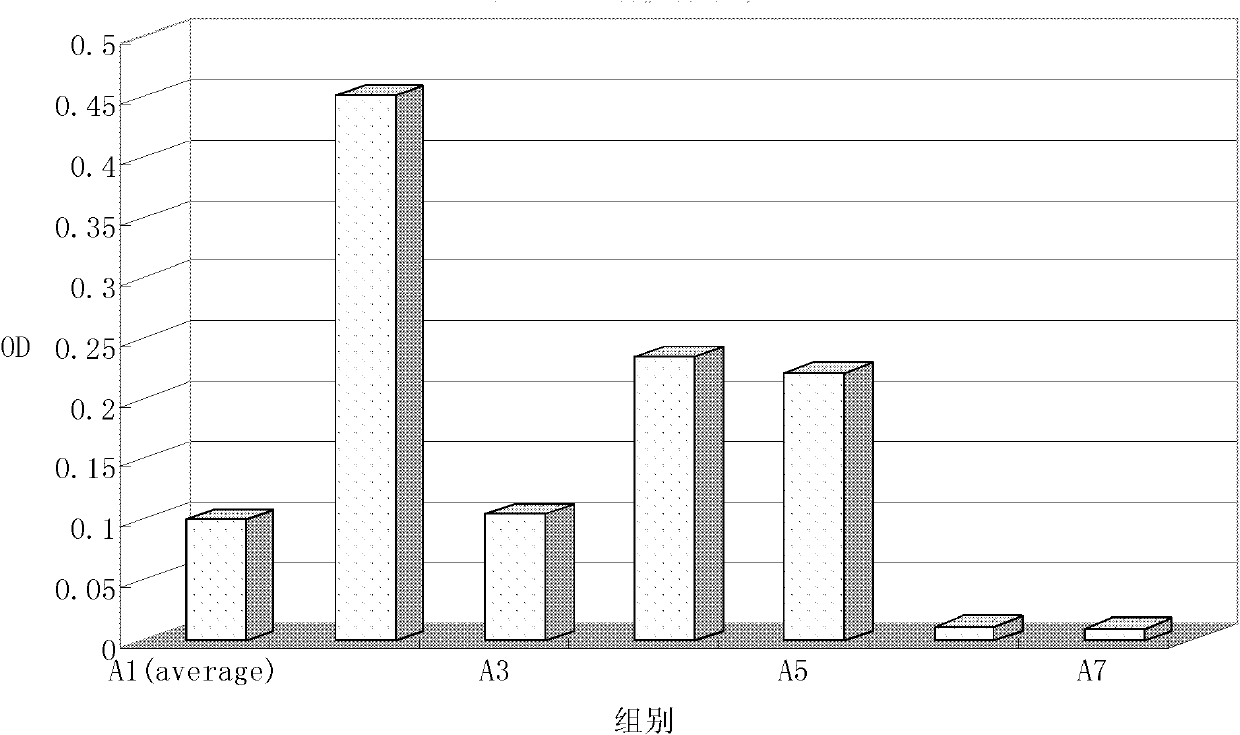
[0040] Example 1 α-mannan peptide adjuvant H1N1 vaccine intramuscular injection and its immune effect detection
[0041] 1.1 Preparation of H1N1 vaccine intramuscular injection and control preparation
[0042] Vaccine solution: Take 40 μL of H1N1 split vaccine solution containing 420 μg / mL of HA, add it to 360 μL of water for injection, and dilute it 10 times to obtain 400 μL of vaccine dilution solution with a concentration of 0.042 μg / μL, which is recorded as drug solution ①. The H1N1 split vaccine was donated by Zhejiang Tianyuan Biological Products Co., Ltd.
[0043] α-mannan peptide solution: Take 15 mg of α-mannan peptide and dissolve it in 1 mL of water for injection to obtain 1 mL of adjuvant solution with a concentration of 15 mg / mL, which is recorded as liquid ②. α-Mannan peptide was purchased from Sinopharm Chuankang Pharmaceutical Co., Ltd.
[0044] The preparation of intramuscular injection of H1N1 vaccine with different proportions is shown in Table 1, in which...
Embodiment 2
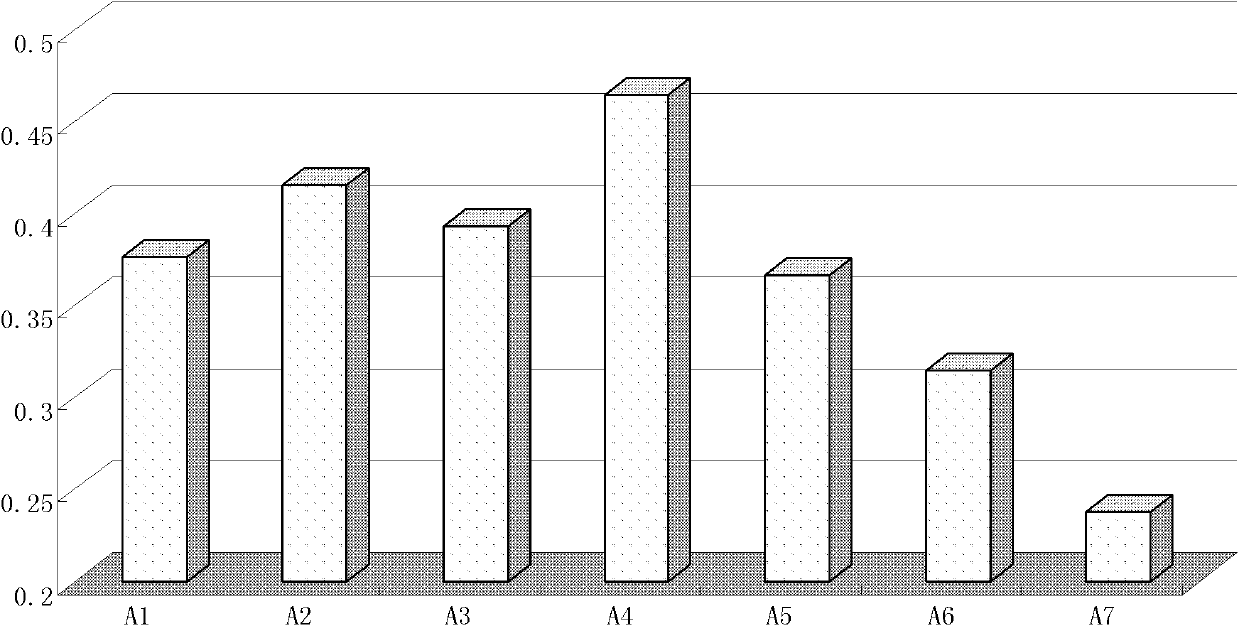
[0078] Example 2. α-mannan peptide adjuvant H1N1 vaccine nasal drops and its immune effect detection
[0079] 2.1 Preparation of H1N1 vaccine nasal drops and control preparations
[0080] 1) Preparation of nasal drop adjuvant solution:
[0081] Sucrose 684mg, sodium glutamate 9.4mg, pig skin collagen 100mg, arginine 121mg, K 2 HPO 4 113mg, KH 2 PO 4 After mixing 48mg, add water to fully dissolve and add water to 5mL.
[0082] 2) Vaccine solution: Take 40 μL of vaccine solution containing 420 μg / mL of HA, add it to 360 μL of water for injection, and dilute by 10 times to obtain 400 μL of vaccine dilution solution with a concentration of 0.042 μg / μL.
[0083] 3) Adjuvant solution: 50 mg of α-mannan peptide was dissolved in 1 mL of water for injection to obtain 1 mL of adjuvant solution with a concentration of 50 mg / mL.
[0084] The preparation of intramuscular injection of H1N1 vaccine with different proportions is shown in Table 1, in which, 1-2 are the vaccine control g...
Embodiment 3
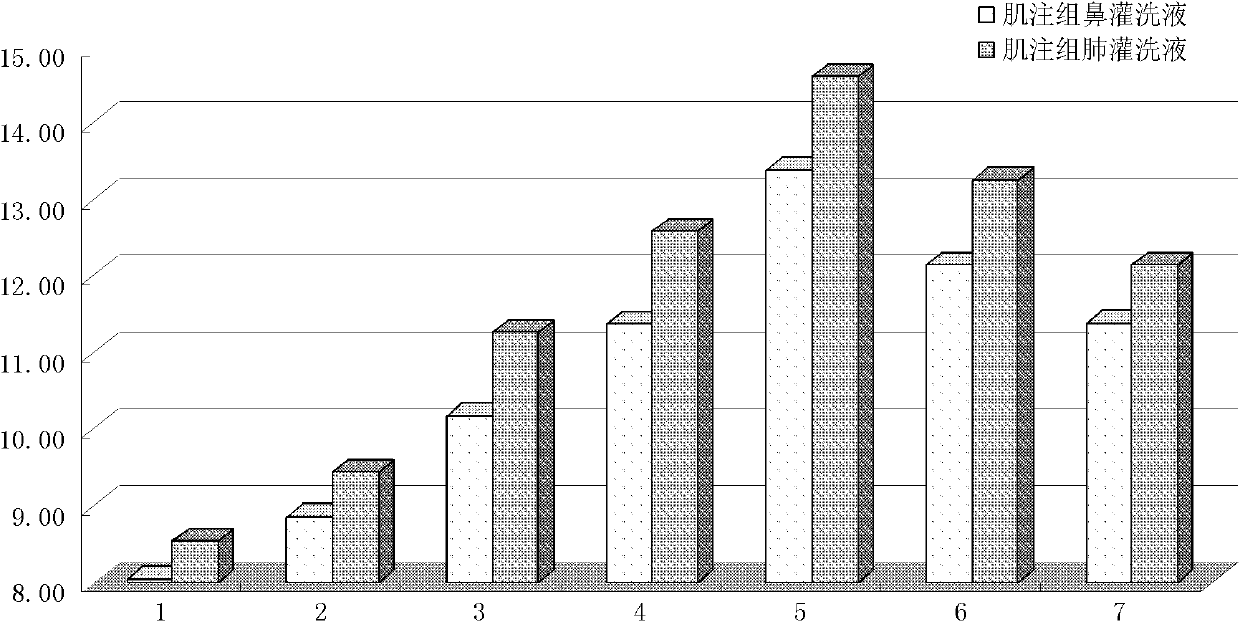
[0114] Example 3 Preparation of Intramuscular Injection of α-Mannan Peptide Adjuvant Seasonal Influenza Vaccine
[0115] Vaccine solution: Take 40 μL of the seasonal influenza trivalent vaccine solution, add it to 360 μL of water for injection, and dilute the amount by 10 times to obtain 400 μL of the vaccine dilution, which is recorded as the drug solution ①.
[0116] Adjuvant solution: Take 15 mg of α-mannan peptide and dissolve it in 1 mL of water for injection to obtain 1 mL of adjuvant solution with a concentration of 15 mg / mL, which is recorded as liquid ②.
[0117] The dosage formula of intramuscular injection of seasonal influenza vaccine is shown in Table 3:
[0118] Table 3 Preparation of intramuscular injection of seasonal influenza vaccine
[0119] group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com