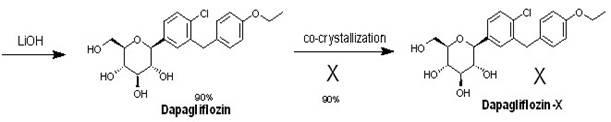
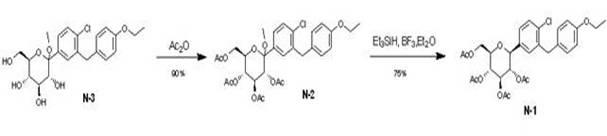
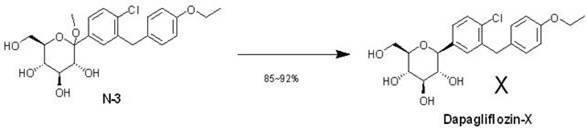
Eutectic preparation method of sodium-glucose cotransporter 2 bulk pharmaceutical chemicals
A technology for glucose and raw materials, which is applied in the field of co-crystal preparation of sodium-glucose cooperating protein-2 raw materials, can solve the problems of low yield of the main product in beta configuration, many reaction steps, and high demethoxylation conditions. It achieves the effects of simple and economical demethoxyl conditions and reagents, shortened reaction steps and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 4 grams of N-3, 50 milliliters of methanol, and 5 milliliters of water into a 250 milliliter three-necked flask, and raise the temperature to 70° C. under stirring to make the suspension gradually transparent. Add 5 g of L-proline and continue stirring for 2 h. Sodium cyanoborohydride was added in batches until all reactions of N-3 were detected by HPLC, and hydrochloric acid was added to adjust the pH value to 7. Then the temperature was lowered and stirred overnight, and a precipitate was precipitated. Filtrate, beat with n-hexane, and dry the solid in vacuum for 24 hours to obtain 5 g of the final product as a white solid (purity 99.62%, yield 85%). 1H NMR (400MHz, DMSO), d12.05 (s, 2H), 7.34 (d, 1H, J=6Hz), 7.32 (m, 2H), 7.08 (d, 2H, J=8.6Hz), 6.78 (d , 2H, J=8.8Hz), 4.10-3.83 (m, 8H), 3.69-3.60 (m, 3H), 3.30 (m, 4H), 3.05 (b, 2H), 2.82 (m, 4H), 1.90- 1.55 (m, 8H), 1.34 (t, 3H, J=7Hz).
Embodiment 2
[0022] In a 250 ml three-neck flask, add 4.4 g of N-3, 60 ml of ethanol, and 6 ml of water, and raise the temperature to 80° C. under stirring to make the suspension gradually transparent. Add 5.5 g of L-proline and continue stirring for 2 h. Sodium acetate borohydride was added in batches until all reactions of N-3 were detected by HPLC, and hydrochloric acid was added to adjust the pH value to 7. Then the temperature was lowered and stirred overnight, and a precipitate was precipitated. After filtration, it was slurried with n-hexane, and the solid was vacuum-dried for 24 hours to obtain 5.8 g of a white solid final product (purity 99.36%, yield 89%).
Embodiment 3
[0024] In a 100 ml three-necked flask, add 2.2 g of N-3, 30 ml of ethanol, and 3 ml of water, and raise the temperature to 80° C. under stirring, so that the suspension becomes gradually transparent. Add 3.4 g of L-tryptophan and continue stirring for 2 h. Sodium cyanoborohydride was added in batches until all reactions of N-3 were detected by HPLC, and hydrochloric acid was added to adjust the pH value to 7. Then the temperature was lowered and stirred overnight, and a precipitate was precipitated. After filtration, it was slurried with n-hexane, and the solid was vacuum-dried for 24 hours to obtain 3.6 g of a white solid final product (purity 99.45%, yield 88%). 1H NMR (400MHz, DMSO), 12.45 (s, 2H), 10.53(s, 2H), 8.33(m, 4H), 7.60 (d, 2H, J=5.5Hz), 7.30-7.06(m, 13H), 6.78 (d, 2H, J=8.8Hz), 4.14-3.90 (m, 9H), 3.85-3.65 (m, 2H), 3.42-3.06 (m, 8H), 1.43(t, 3H, J=6.8Hz) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com