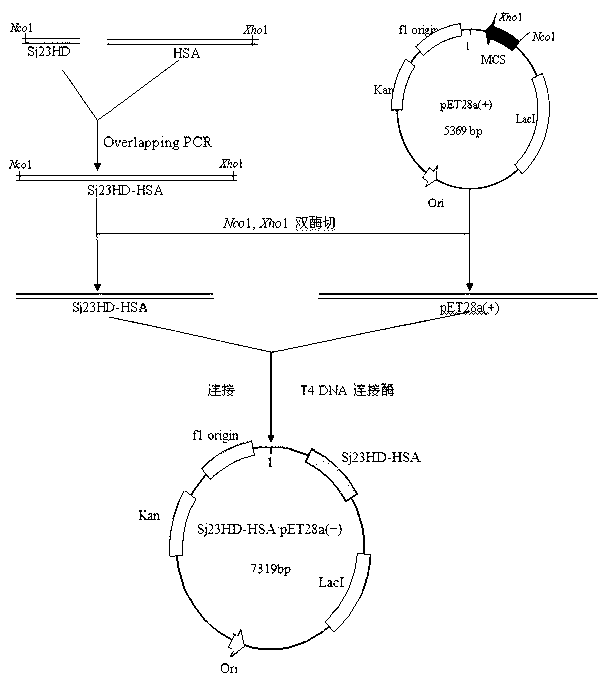
Schistosoma japonicum 23kDa membrane protein big hydrophilic peptide segment fusion protein and application thereof in schistosome infection immune diagnosis
A fusion protein and membrane protein technology, applied in the direction of peptides, hybrid peptides, specific peptides, etc., can solve the problems of insufficient specificity and early diagnostic value, achieve good diagnostic value of schistosomiasis, improve stability, improve sensitivity and specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: human serum albumin gene cloning
[0068] The human serum albumin gene is reverse-transcribed and synthesized from the mRNA of human fetal liver tissue by RT-PCR technology. The specific preparation method is as follows:
[0069] 1. Preparation of human fetal liver mRNA
[0070] Take 0.5 g of freshly isolated human fetal liver tissue, freeze it in liquid nitrogen, pulverize it in a ceramic mortar, and use the mRNA purification kit (Illustra QuickPrep TM mRNA purification kit) was used to prepare and purify mRNA, and the operation method was strictly in accordance with the operation instructions of the kit. The purity and content of mRNA were measured by UV spectrophotometer.
[0071] 2. HSA gene amplification
[0072] 2.1 Primer design:
[0073] HSA1: 5′-GATGCACACAAGAGTGAGGT-3′
[0074] HSA2: 5'-AACTCGAGTTATAAGCCTAAGGCAGCTTGACTTGC-3'.
[0075] 2.2 First strand cDNA synthesis
[0076] Using Phusion TM RT-PCR Kit (purchased from NEB Beijing Branch)...
Embodiment 2
[0079] Example 2: Sj23HD gene synthesis
[0080] The Sj23HD gene is prepared by artificial synthesis. In order to facilitate the fusion of the Sj23HD gene and the HSA gene, and to facilitate gene cloning, a Nco 1, the 5' end DNA sequence of part of the HSA gene is carried at its 3' end. The synthesized Sj23HD gene is shown as SEQ ID NO:5. The synthesis of the gene sequence was completed by Shanghai Handsome Biotechnology Co., Ltd.
Embodiment 3
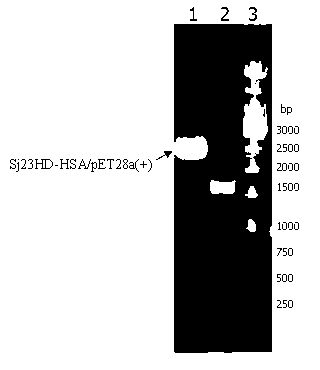
[0081] Example 3: Construction and sequence analysis of Sj23HD-HSA fusion protein gene
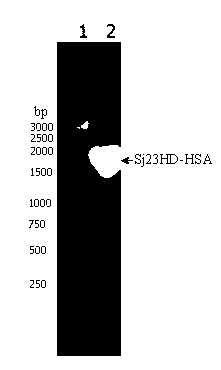
[0082]The preparation of the Sj23HD-HSA fusion protein gene is carried out according to the principle of Overlapping PCR.
[0083] Primer design:
[0084] Sj23HD1: 5′-CATGGATGACTGGTGCTCT-3′,
[0085] HSA2: 5'-AACTCGAGTTATAAGCCTAAGGCAGCTTGACTTGC-3'.
[0086] Gene Amplification:
[0087] In a 0.2mL PCR tube, add 2×Phusion Master Mix 25μL, synthetic Sj23HD gene fragment 2μL, purified HSA gene fragment 2μL (the molecular ratio of these two gene fragments should be adjusted to 1:1 as much as possible), add no Ionized water to a final volume of 48 μL, 98°C for 30 Sec; anneal at 65°C for 30 Sec, extend at 72°C for 2 min, and cool the reaction tube on ice. Add 1 μL each of primers Sj23HD1 and HSA2, mix well, and centrifuge to collect the reaction product at the bottom of the tube. Then carry out gene amplification according to the following conditions: 98°C, 10 Sec; 65°C, 20 Sec, 72°C, 50 Sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com