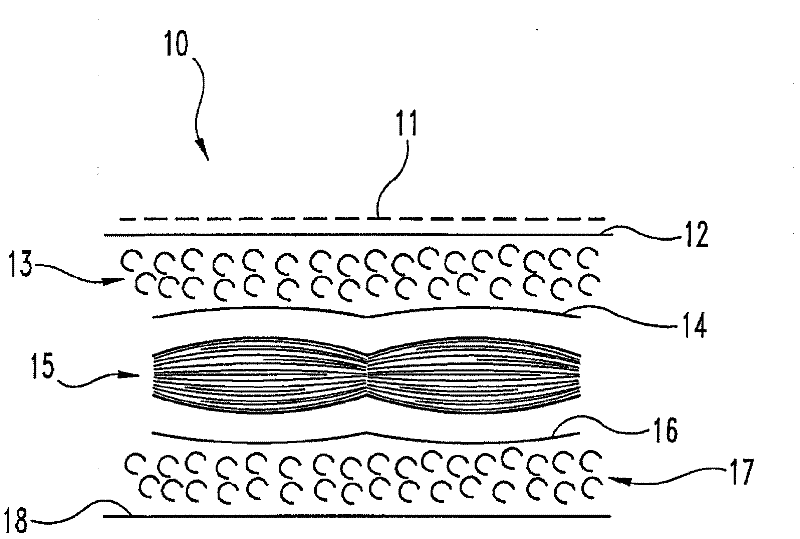
Isolated extracellular matrix material including subserous fascia
A technology of extracellular matrix and cells, applied in the field of medical materials, which can solve the problems of limited size and strength of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of extracellular matrix graft material
[0048] In a food processing plant, tissue fragments are collected at points on the processing line, a midline incision is used at the processing line to open a complete porcine body cavity, and the front ribs and all organs are removed. An incision was made on the back edge of the body wall flap on each side of the body, and the tissue fragments were removed on each side of the body wall to make two tissue fragments of approximately equal dimensions (1 foot x 1.5 feet). These fragments include the peritoneum, subserosal fascia, and subserosal fat. It was determined that the posterior fascia was not part of the tissue fragment. Depending on the location along the segment, the subserosal fat is about 0.5 to about 1 cm thick. Place the peritoneum containing both the visceral peritoneum and the parietal peritoneum. The visceral peritoneum was discarded, which was determined to be a transparent film with a thick white netw...
Embodiment 2
[0053] Test natural lipid content
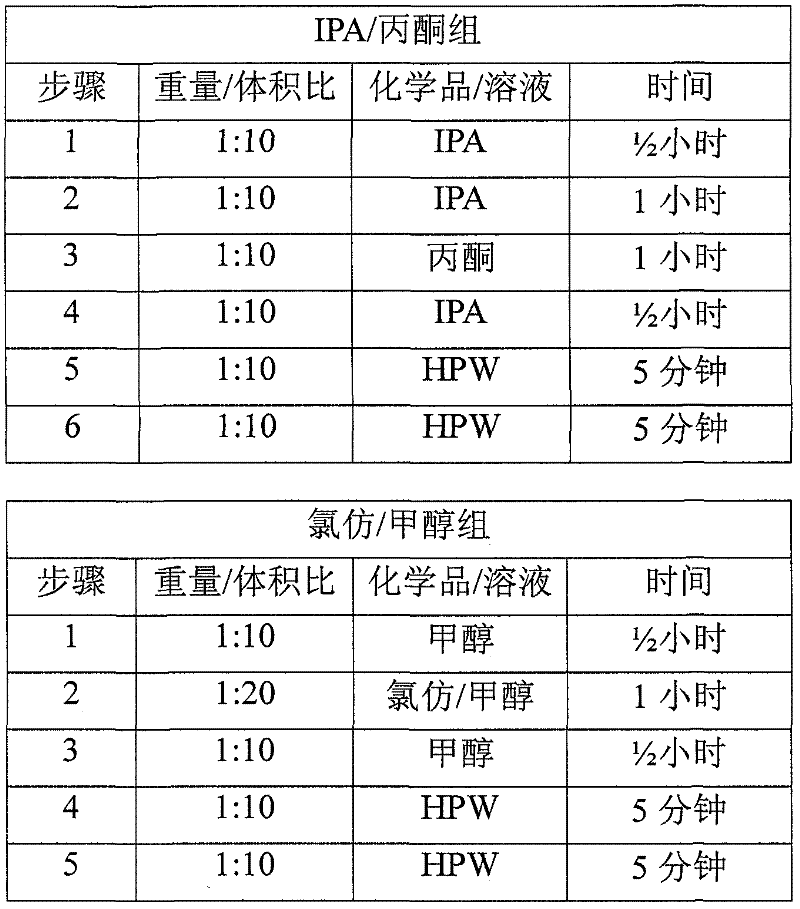
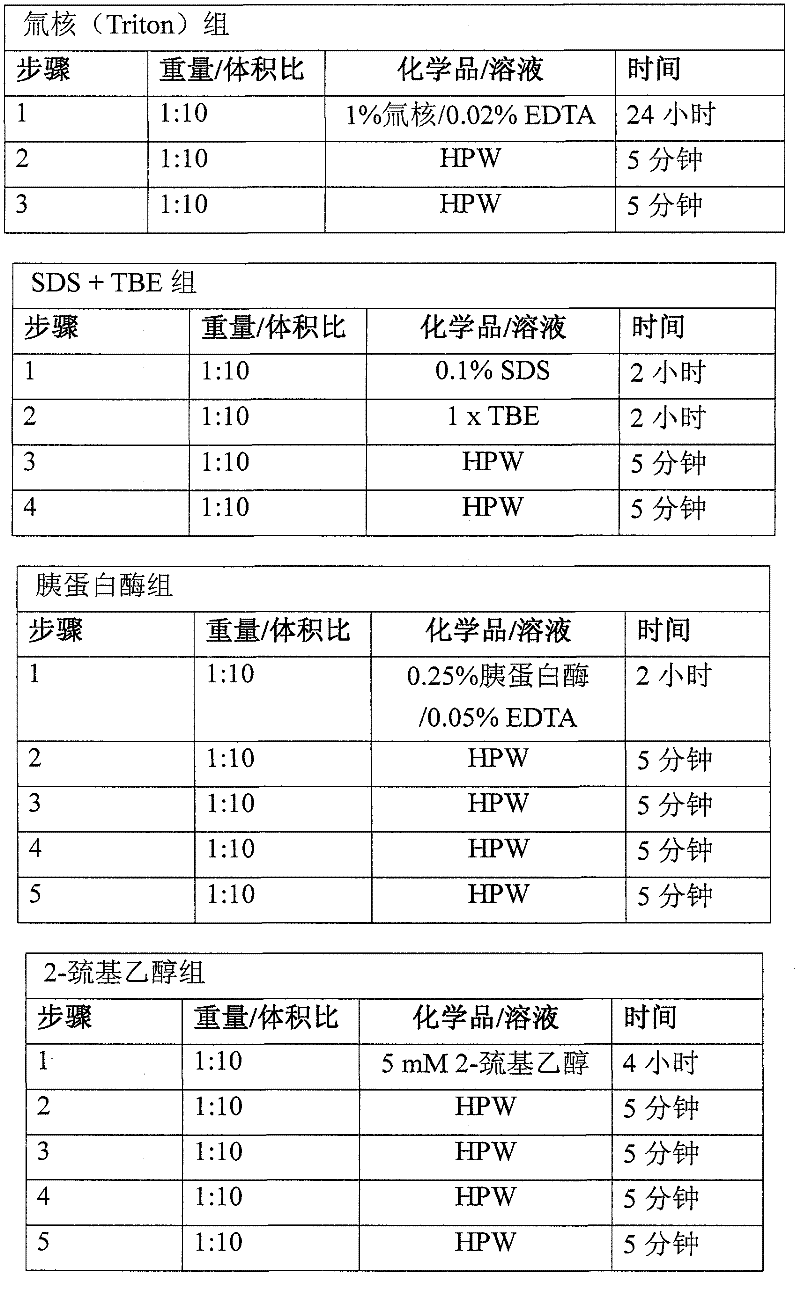
[0054] Usually 2 batches of material are obtained as described in Example 1. The abdominal wall tissue fragments were processed by mechanical scraping, treatment with 100% isopropanol (IPA) (1:10 weight: volume) three times, disinfection with peracetic acid (PAA) solution, and washing four times with high-purity water to process abdominal wall tissue fragments. Each batch consists of 2 pieces, and a total of four pieces are tested. Cut four 4x7 cm samples from each piece to obtain 8 samples per batch for a total of 16 samples. Each sample was weighed (initial weight), rolled and placed in a 1.5 ml centrifuge tube with 10 ml of 100% ethanol. Place the tube in a tube holder and place the holder on the side of a rotary shaker at room temperature for approximately 24 hours. After 24 hours, the ethanol was drained and 10 ml of 100% acetone was placed in each tube. The rack was then placed back on the shaker for approximately 24 hours at room tem...
Embodiment 3
[0057] Test the content of natural hyaluronic acid
[0058] Usually 2 batches of material are obtained as described in Example 1. The abdominal wall tissue fragments were processed by mechanical scraping, treatment with 100% isopropanol (IPA) (1:10 weight: volume) three times for chemical degreasing, disinfection with peracetic acid (PAA) solution, and washing four times with high-purity water. Each batch consists of 2 pieces, and a total of four pieces are tested. Cut four 12 mm disc-shaped samples from each piece to obtain 8 samples per batch, for a total of 16 samples. Each sample was weighed (initial weight), rolled and placed in a 1.5 ml centrifuge tube with 450 microliters of sterile phosphate buffered saline (PBS) and 50 microliters of proteinase K at 56°C for 45 minutes. The samples were then crushed with a tissue grinder for 90 seconds per sample. The tube with the sample was centrifuged at 12000g for 5 minutes at 4°C to pellet any undigested material. A 1:40 dilutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com