Structure of coxsackie virus A16-3C protease and application thereof
A coxsackie virus and protease technology, applied in the field of 3C protease structure, can solve the problem of difficult screening of specific inhibitor molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The acquisition of embodiment 1 CVA16 3C protease
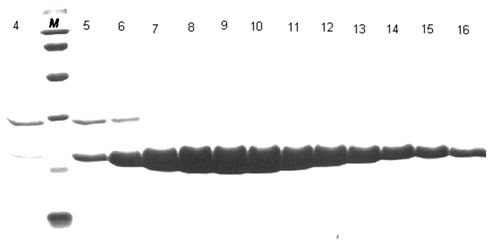
[0033] We successfully expressed the 3C protease of CVA16 virus in vitro, and obtained high-purity and active enzyme protein by affinity chromatography and molecular exclusion chromatography.
[0034] The amino acid sequence of CVA16 3C protease is shown in SEQ ID NO.1, or the sequence shown in SEQ ID NO.1 is substituted, deleted or added with one or several amino acids and has 3C protease activity derived from the amino acid sequence of SEQ ID NO.1 of protein.
[0035] Protein sequence SEQ ID NO.1:
[0036] GPSLDFALSLLRRNIRQVQTDQGHFTMLGVRDRLAILPRHSQPGKTIWVEHKLINVLDAVELVDEQGVNLELTVTLDTNEKFRDVTKFIPETITGASDA
[0037] 1. Plasmid construction:
[0038] We artificially synthesized the DNA fragment encoding 3C protease, ligated it into the pET-21a vector with Nde1 and Xho1 restriction sites, and inserted a 6-histidine tag (hexa-His-tag) behind the encoding fragment of the enzyme protein. The coding sequence is followed ...
Embodiment 2
[0050] Example 2 Structure of CVA16 3C protease
[0051] 1. Protein crystallization conditions:
[0052] We used the hang-drop vapor-diffusion method and optimized the conditions to obtain crystals with good diffraction quality.
[0053] The protein preparation solutions used for crystallization all have a protein concentration of 10 mg / ml. The conditions are as follows:
[0054] CVA16-3C crystallization conditions: 0.1M BIS-TRIS pH 5.5, 0.1M ammonium acetate and 17% w / v PEG 10000
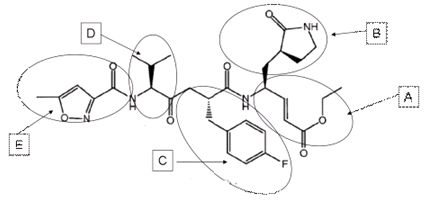
[0055] The crystallization conditions of the complex of CVA16-3C-C147A and FAGLRQAVTQ polypeptide: 0.1 M HEPES pH7.5, 0.2M lithium sulfate and 25% w / v PEG 3350, the molar concentration ratio of protein to polypeptide is 1:5.
[0056] The crystallization conditions of the complex of CVA16-3C and AG7088: 0.1 M sodium acetate pH4.6, 0.1M magnesium chloride and 25% w / v PEG 4000, the molar concentration ratio of protein to compound is 1:3.
[0057] 2. Data collection and structure analysis:
[...
Embodiment 3
[0062] Example 3 The substrate binding groove of CVA16-3C protease
[0063] The nomenclature of each site of the 3C protease substrate binding groove: usually we use the peptide bond cleaved on the substrate polypeptide as the reference point, and the amino acids to the left are named P1, P2, P3, P4,...Pn in sequence, and the amino acids to the right are named in sequence Named P1', P2', P3', P4',...Pn'; and the part of the protease that accommodates these polypeptide amino acids is correspondingly named as the site S1, S2, S3, S4,...Sn, S1 ', S2', S3', S4',...Sn'.
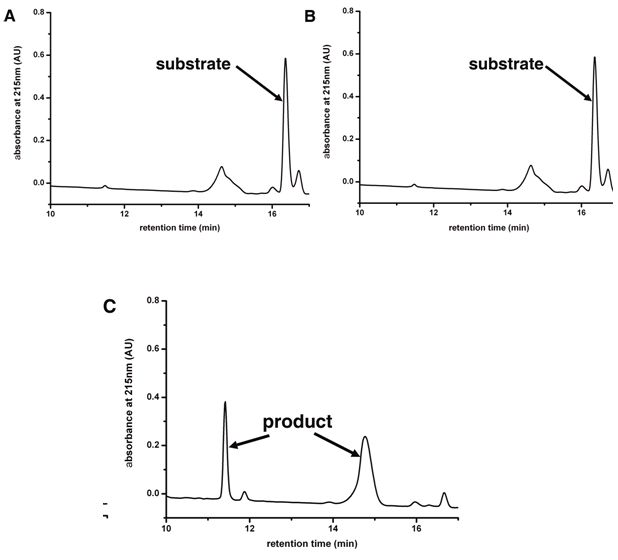
[0064] Through the complex structure of the substrate polypeptide and CVA16-3C, and the complex structure of the compound AG7088 and CVA16-3C, we were able to determine the conserved substrate-binding groove in proteases ( Figure 5 ), Figure 5 The stick model in A is the substrate polypeptide, Figure 5 The stick model in B is the compound AG7088, both of which are combined in the same groove-like structure on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com