Application of recombinant human Rho kinase to preparation of medicaments
A technology of kinase inhibitors and drugs, which is applied in the application field of recombinant human Rho kinase in the preparation of drugs, and can solve the problem that recombinant human ROCK1 functional fragment protein has not been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
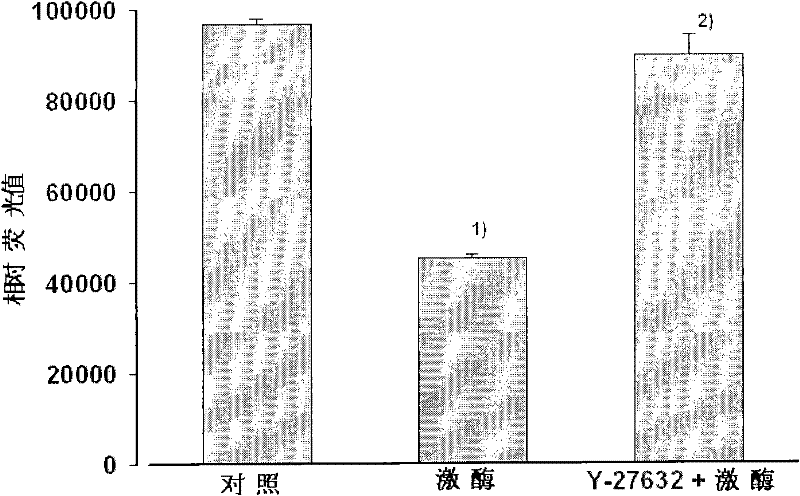
[0024] Example 1: Evaluation of Kinase Biological Activity of RP1.
[0025] In this example, the activity of RP1 was identified by the Kinase-Glo Luminescent Kinase Assay method. The reaction was carried out in a white opaque 96-well plate, and 5 μL of kinase buffer (25 mmol L -1 Tris-HCl, pH7.5, 10mmol L -1 MgCl 2 , 0.1mg·mL -1 BSA), 10 μL kinase, 10 μL S6 (AKRRLSSLRA) polypeptide substrate and 5 μL ATP. After mixing, incubate at 37°C for a certain period of time, add 50 μL of Kinase-Glo reagent, room temperature for 10 min, and read the relative luminescence value of each well on SpectraMax M5. Add 10 μmol·L to the reaction -1 Y-27632 (a specific inhibitor of Rho kinase), observe whether it can selectively inhibit the activity of RP1, and compare it with the blank control without kinase and the positive control that adds kinase at the same time, to determine the activity of RP1 in Example 1 Rho kinase biological activity.
[0026] There is a significant difference be...
Embodiment 2
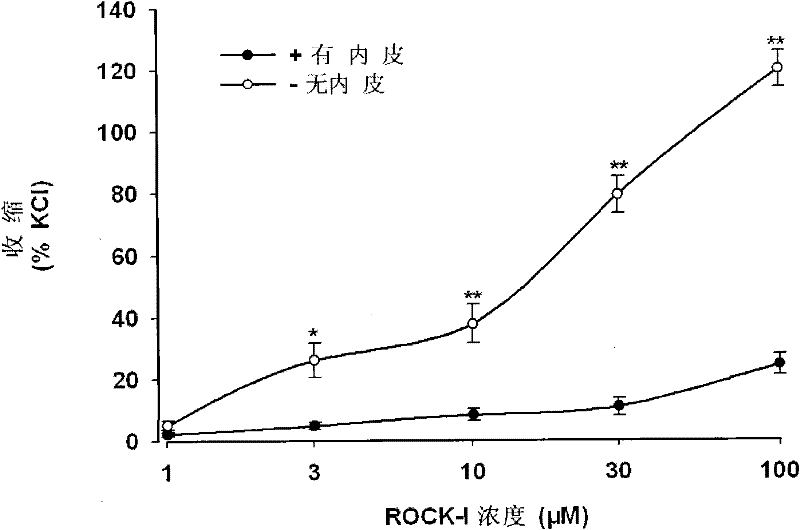
[0027] Example 2: Effect of RP1 on vascular reactivity in anesthetized rats.
[0028] The experimental animals in this example were male SD rats (body weight 250-300 g). After the rats were killed by decapitation, the thoracic aorta was quickly taken out, cut into 2-3mm long vascular rings, and placed in a 10mL K-H solution (37°C constant temperature, and continuously fed with 95% oxygen and 5% carbon dioxide). In the bath of mixed gas), the tension change is transmitted and recorded in the BL-420S biological function experiment system. The vascular ring was stabilized at 1.2g tension for 60 minutes, and the K-H solution was changed every 20 minutes during this period. Stimulate the vascular ring with 60mM KCl to shrink the vascular ring. After reaching the maximum amplitude, flush the vascular ring twice to restore the vascular ring to the state before the stimulation, a total of 2 times. Add 1 μM norepinephrine, give 10 μM acetylcholine after reaching the maximum contracti...
Embodiment 3
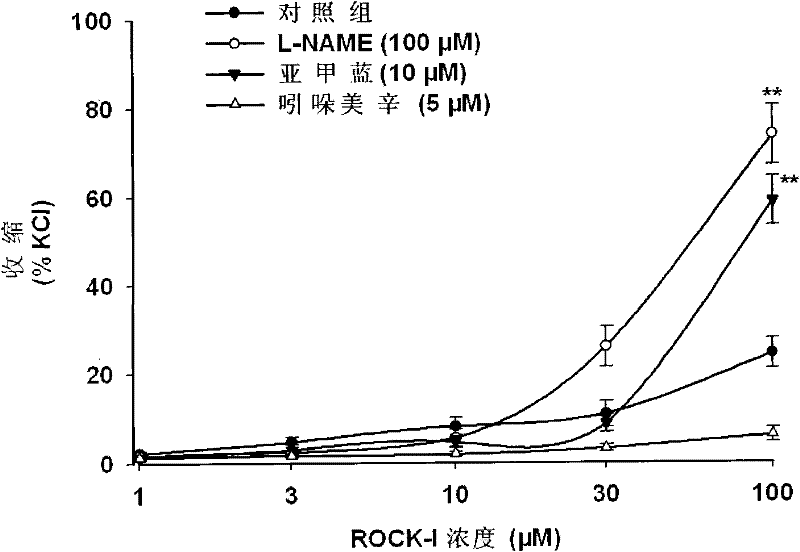
[0030] Example 3: Effects of various endothelial-derived active substances on the vasoconstrictor effect of RP1.
[0031] The preparation method of the blood vessel ring for tension measurement is the same as that in Example 3.
[0032]In this example, nitric oxide synthase inhibitor L-NAME (100 μM), cyclooxygenase inhibitor indomethacin (5 μM), guanylate cyclase inhibitor methylene blue (10 μM) were added to the reaction system ), adding cumulative concentration of RP1 after pre-incubation for 20 min to observe the effect of endothelial-derived active substances on the vasoconstrictor effect of RP1. L-NAME can significantly enhance the vasoconstrictor effect of RP1 (attached image 3) . It was determined that the regulation of RP1 on vascular function was endothelium-dependent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com