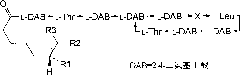Polymyxin E2 composition, and preparation method and application thereof
A technology of colistin and composition, which is applied in the field of polymyxin E2 composition, and can solve the problems of unproven colistin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: polymyxin E sulfate 2 Preparation of the composition
[0029] Weigh an appropriate amount of industrial-grade polymyxin E sulfate, add water to dissolve, and make a solution containing 100 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0030] Column: C 18 SunFire Prep OBD (10μm); 19×150mm, purchased from WATERS, USA
[0031] Column volume (CV): 42.5ml
[0032] Flow rate: 10.0ml / min
[0033] Elution conditions: Use 30% ethanol-containing dilute sulfuric acid solution (use 0.05mol / L sulfuric acid to adjust the pH of the solution to 2.3.) Isocratic elution for 30CV.
[0034] Sample volume: 50ml
[0035] Collection: 10ml / tube
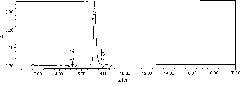
[0036] Analysis and processing of collected samples: analyzed by analytical HPLC (chromatographic conditions refer to the determination of the purity of polymyxin E in European Pharmacopoeia 5.0), and the combined polymyxin sulfate with a purity of more than 95% 2 The e...
Embodiment 2
[0038] Embodiment 2: polymyxin E sulfate 2 Preparation of the composition
[0039] Weigh an appropriate amount of industrial-grade polymyxin E sulfate, add water to dissolve, and make a solution containing 120 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0040] Chromatography packing: polystyrene / divinylbenzene as the skeleton and bonded phenyl, model NM-100, produced by Suzhou Nano Microbe Technology Co., Ltd.
[0041] Column specification: 45×400mm,
[0042] Column volume (CV): 635.9ml
[0043] Flow rate: 30.0ml / min
[0044] Elution conditions: first use 25% ethanol sulfuric acid dilute solution (use 0.05mol / L sulfuric acid to adjust the pH value of the solution to 2.3.) to elute 20CV, and then use 30% ethanol sulfuric acid dilute solution (use 0.05mol / L sulfuric acid to Adjust the pH value of the solution to 2.5.) Elution 20CV.
[0045] Sample volume: 500ml
[0046] Collection: 100ml / tube
[0047] Analysis...
Embodiment 3
[0049] Embodiment 3: polymyxin E sulfate 2 Preparation of the composition
[0050] Weigh an appropriate amount of industrial-grade polymyxin E sulfate, add water to dissolve, and make a solution containing 120 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0051] Chromatography packing: polystyrene / divinylbenzene as the skeleton and bonded phenyl, model NM-100, produced by Suzhou Nano Microbe Technology Co., Ltd.
[0052] Column specification: 25×200cm,
[0053] Column volume (CV): 98.13L
[0054] Flow rate: 200.0ml / min
[0055] Elution conditions: first use 23% ethanol sulfuric acid dilute solution (use 0.05mol / L sulfuric acid to adjust the pH value of the solution to 2.3.) to elute 26CV, and then use 30% ethanol sulfuric acid dilute solution (use 0.05mol / L sulfuric acid to Adjust the pH value of the solution to 2.5.) Elution 25CV.
[0056] Sample volume: 100L
[0057] Detection wavelength: 215nm
[0058] Coll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com