Group of siRNA molecules capable of effectively reducing PRDM1beta expression and application thereof
A PRDM1, molecular technology, applied in the direction of recombinant DNA technology, DNA / RNA fragments, medical preparations containing active ingredients, etc., can solve the problem of tumor cell insensitivity to chemotherapy drugs, to overcome drug resistance of lymphoma, improve treatment Efficacy, Enhancement, and Prognostic Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
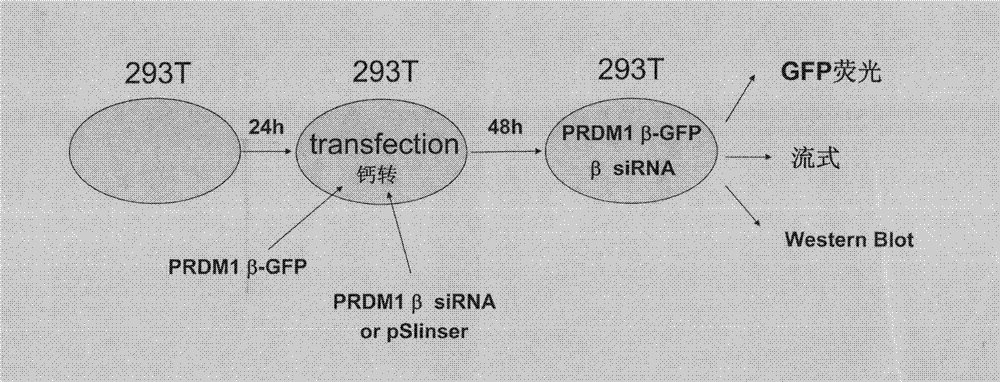
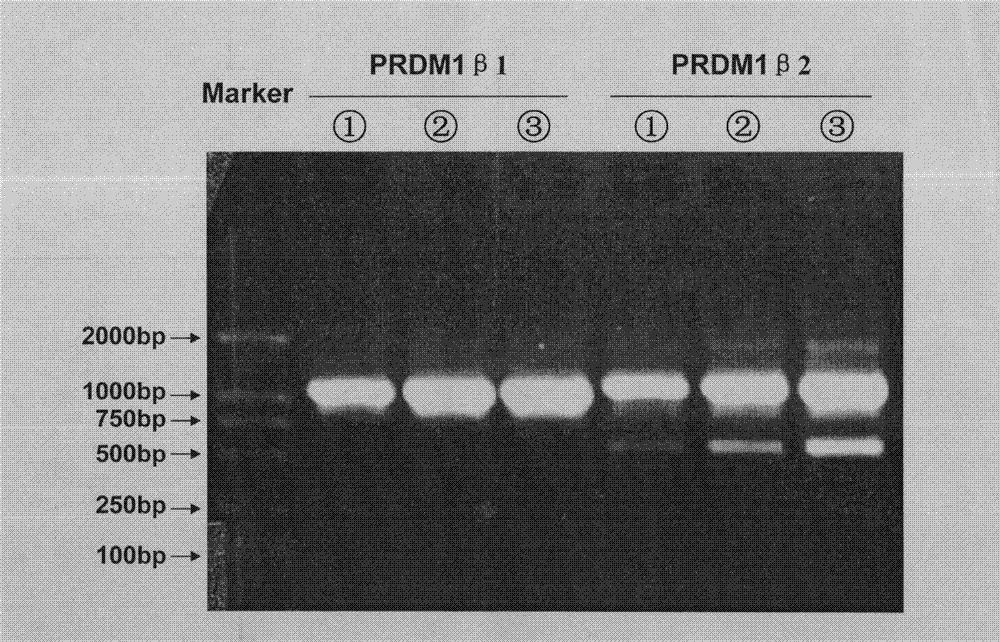
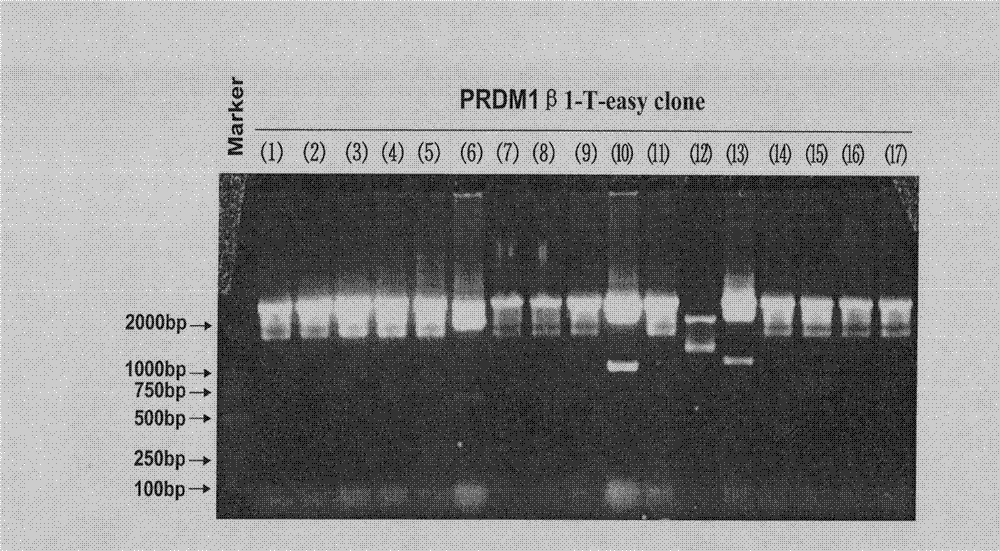
[0039] The specific embodiments provided by the present invention will be described in detail below in conjunction with the accompanying drawings.
[0040] (1) Cell lines and cell culture
[0041] The multiple myeloma cell line 8226, the T-cell lymphoma cell line H9, and the B-cell lymphoma cell line Namalwa were all purchased from the American Standard Biological Collection (ATCC). All cells were cultured in 1640 medium containing 10% fetal bovine serum in 5% CO 2 -95% air, saturated humidity and cultured at 37°C. The survival rate of the cells was detected by the Moss blue exclusion test, and the cell morphology was observed by the Wright's staining method.
[0042] (2) Reagents
[0043] RNA extraction reagent Trizol and RNA reverse transcription kit were purchased from Invitrogen, pEGFP-N2Vector was purchased from BD Biosciences Clontech, pSlencer 4.1-H1 neo Vector was purchased from Ambion, KOD Plus high-fidelity DNA polymerase was purchased from TOYOBO, T-easy was pur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com