Preparation method and application of camphor manganese porphyrin
A technology of camphor manganese porphyrin and camphorone acid chloride, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of low yield and long reaction time, and achieve The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
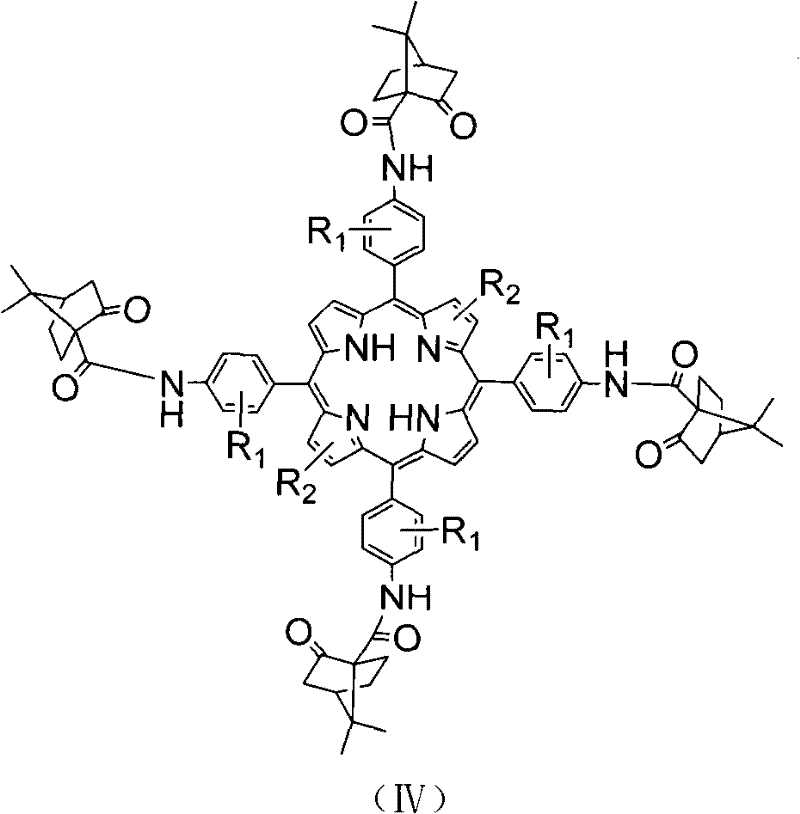
[0023] Take preparation 5,10,15,20-tetrakis (4-camphor ketone amido phenyl) manganese porphyrin as example, used raw material and preparation method thereof are as follows:
[0024] 1. Mix 3.6g (20mmol) camphoronic acid with 2.2mL (30mmol) SOCl 2 Mix, stir at room temperature for 2 hours, reflux at 80°C for 1 hour, distill off SOCl 2 , prepared into camphorone acid chloride, the yield was 95%.
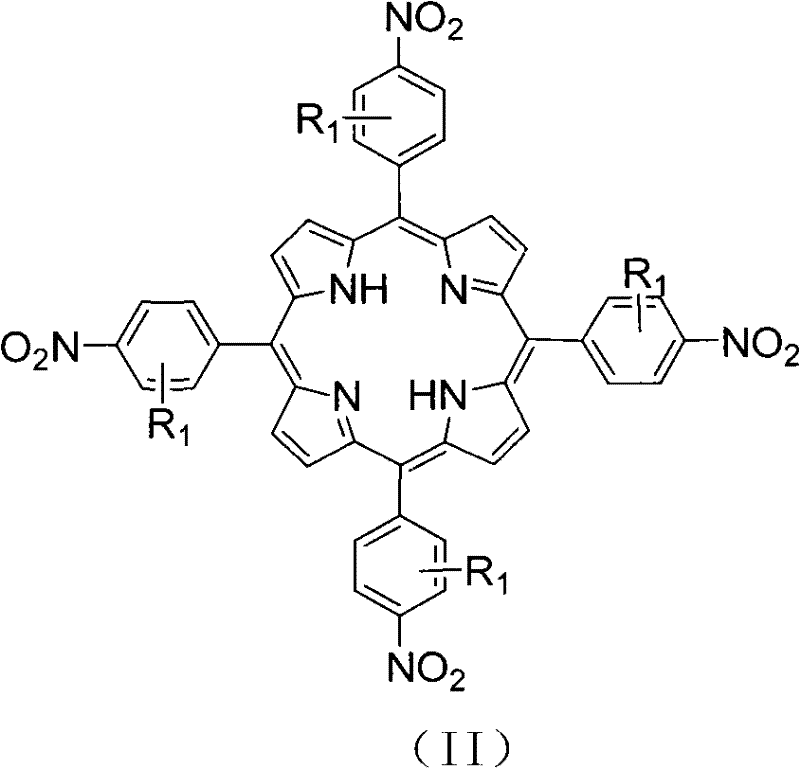
[0025] 2. Mix 11.0g (72mmol) of 4-nitrobenzaldehyde and 5mL (72mmol) of pyrrole and react at 150°C for 0.5 hours to prepare 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin, Its yield was 23.4%.
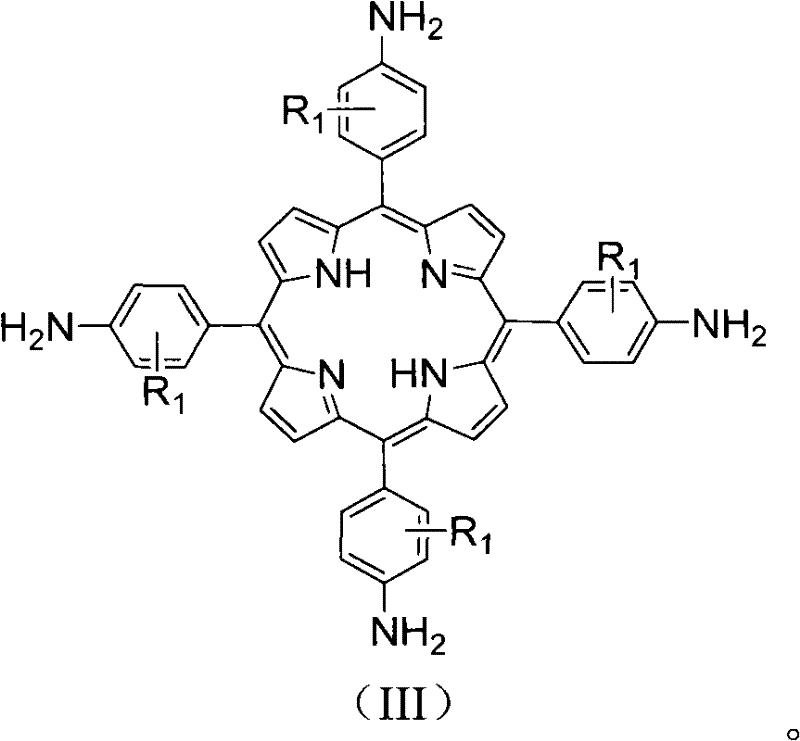
[0026] 3. Add 0.79g (1mmol) 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin and 3.04g (16mmol) stannous chloride to 40mL of 36.5% hydrochloric acid, mix well , reacted at 80° C. for 0.5 hour to prepare 5,10,15,20-tetrakis(4-aminophenyl)porphyrin with a yield of 90%.
[0027] 4. In N 2 Protection, under the condition of -20~-17°C, add 0.63g (1mmol) 5,10,15,20-tetrakis(4-aminophenyl)porphyrin, 1.6g...
Embodiment 2
[0030] Taking the preparation of 2,3,12,13-tetrabromo-5,10,15,20-tetrakis(4-camphoronamidophenyl)manganese porphyrin as an example, the raw materials used and the preparation method thereof are as follows:
[0031] l, 3.6g (20mmol) camphoronic acid and 2.2mL (30mmol) SOCl 2 Mix, stir at room temperature for 2 hours, reflux at 80°C for 1 hour, distill off SOCl 2 , prepared into camphorone acid chloride, the yield was 95%.
[0032] 2. Mix 11.0g (72mmol) of 4-nitrobenzaldehyde and 5mL (72mmol) of pyrrole and react at 150°C for 0.5 hours to prepare 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin, Its yield was 23.4%.
[0033] 3. Add 0.79g (1mmol) 5,10,15,20-tetrakis(4-nitrophenyl)porphyrin and 3.04g (16mmol) stannous chloride to 40mL of 36.5% hydrochloric acid, mix well , reacted at 80° C. for 0.5 hour to prepare 5,10,15,20-tetrakis(2-methyl-4-aminophenyl)porphyrin with a yield of 90%.
[0034] 4. In N 2 Protection, under the condition of -20~-17°C, add 0.63g (1mmol) 5,10,15,20-t...
Embodiment 3
[0038] In step 2 of Example 1, the raw material 4-nitrobenzaldehyde used is replaced by equimolar 3-methoxy-4-nitrobenzaldehyde, and equimolar 3-ethoxy-4-nitrobenzaldehyde can also be used Benzaldehyde or 3-propoxy-4-nitrobenzaldehyde or 3-butoxy-4-nitrobenzaldehyde or 3-methyl-4-nitrobenzaldehyde or 3-ethyl-4-nitro Benzaldehyde or 3-propyl-4-nitrobenzaldehyde or 3-butyl-4-nitrobenzaldehyde are replaced, and other steps of this step are identical with embodiment 1. The other steps are the same as in Example 1, and 5,10,15,20-tetrakis(3-methoxy-4-camphoronamidophenyl)manganese porphyrin or 5,10,15,20-tetrakis(3 -Ethoxy-4-camphoronamidophenyl) manganese porphyrin or 5,10,15,20-tetrakis(3-propoxy-4-camphoronamidophenyl)manganese porphyrin or 5,10 , 15,20-tetrakis(3-butoxy-4-camphoronamidophenyl) manganese porphyrin or 5,10,15,20-tetrakis(3-methyl-4-camphoronamidophenyl) Manganese porphyrin or 5,10,15,20-tetrakis(3-ethyl-4-camphoronamidophenyl)manganese porphyrin or 5,10,15,20-t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com