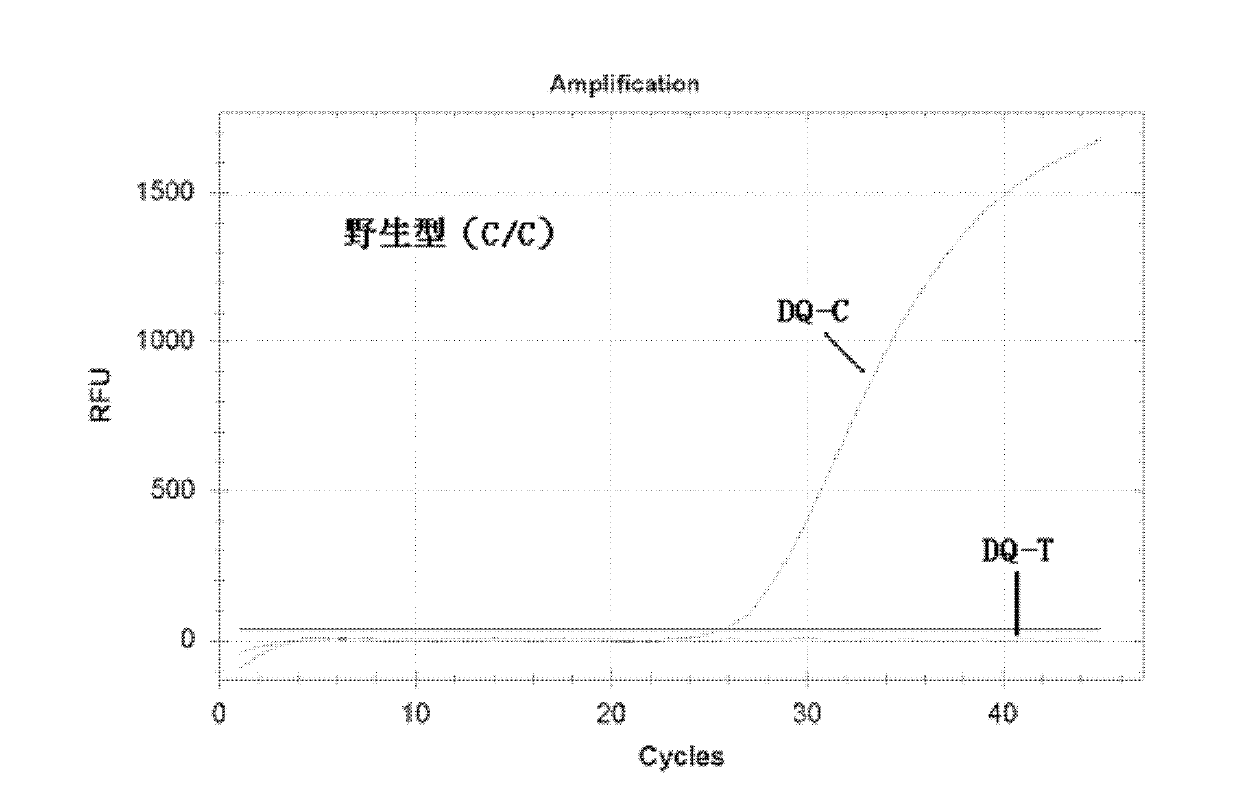
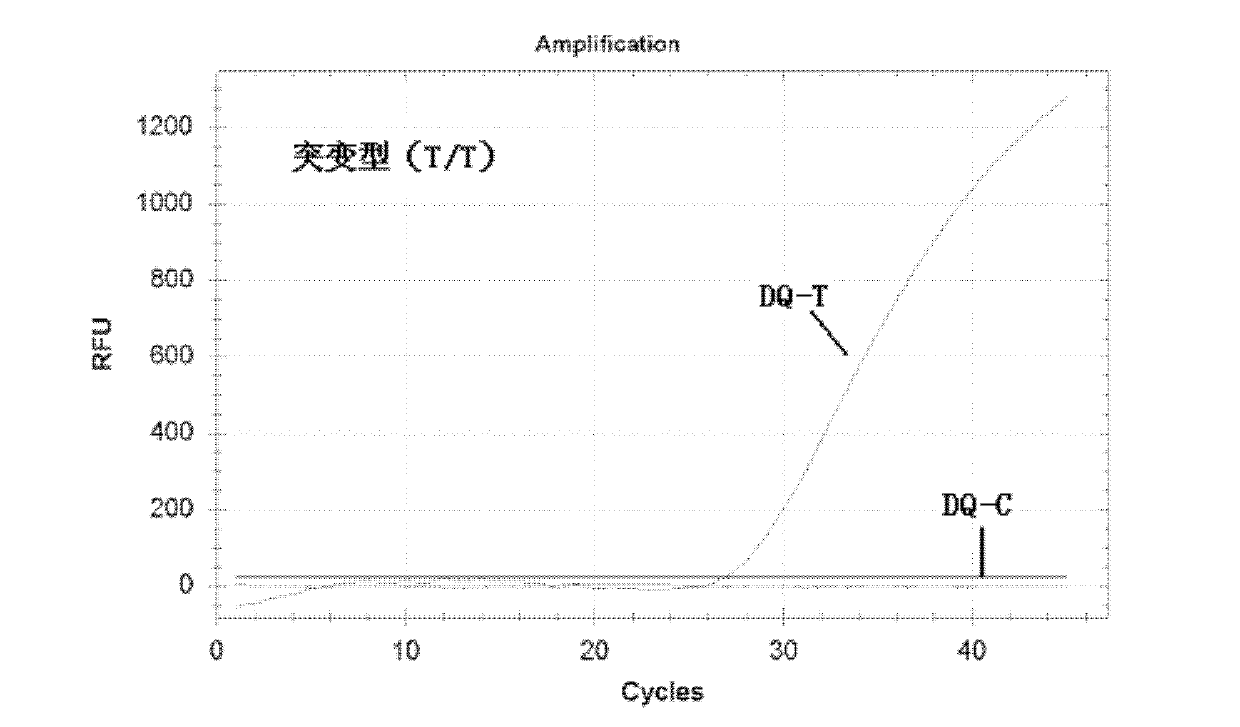
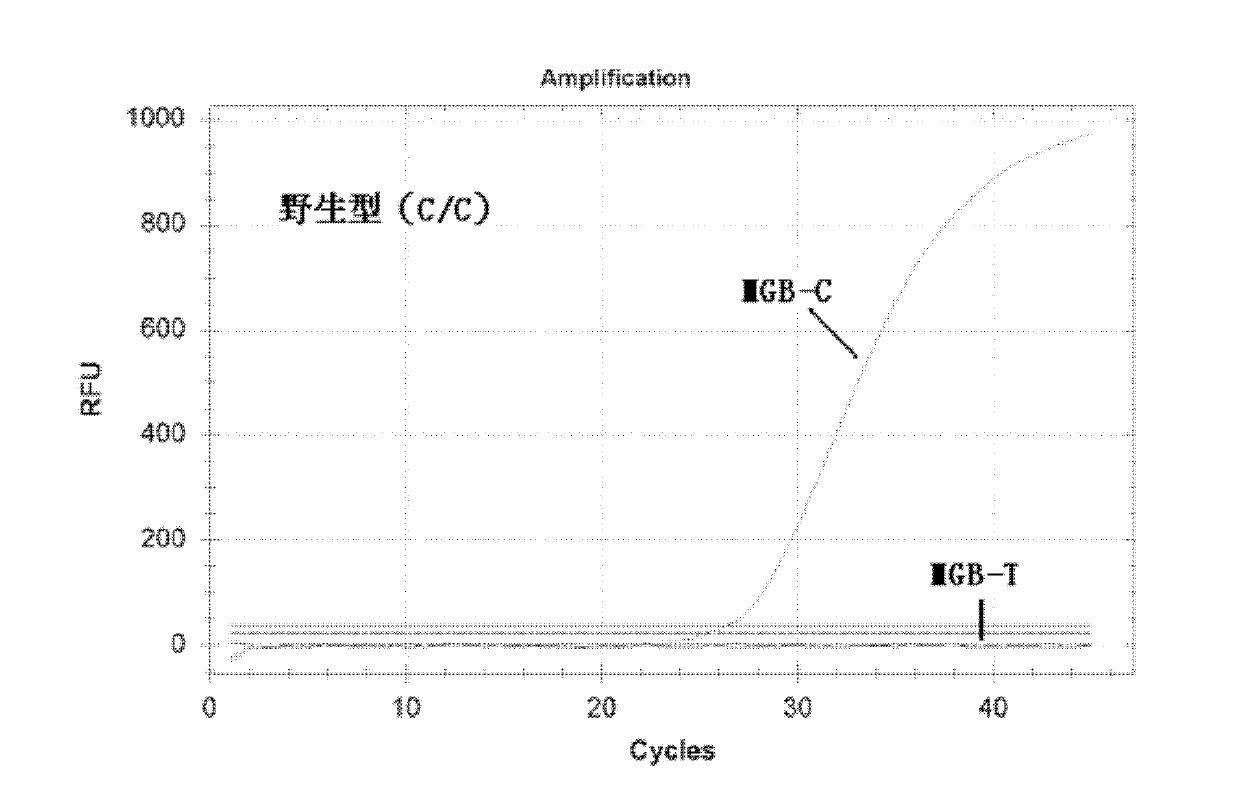
Nucleotide monomer capable of improving Tm value of oligonucleotide chain as well as preparation method and application thereof
A technology of nucleotide monomers and oligonucleotides, applied in the field of nucleotide monomers, can solve the problems of single structure, not high flexibility, MGB probes are not suitable for multiple fluorescence quantitative detection, etc., and achieve flexible modification methods. , the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of DNA minor groove binder DQ
[0043] 1. Synthesis and purification of MGB modified dT:
[0044] Dissolve 5 mg (8.02 μmol) of DPI2 (see compound 2 in the following reaction formula, purchased from Shanghai Huirui Biotechnology Co., Ltd.) in 3ml DMF (dimethylformamide) and 3mL 0.1M NaHCO3, add 3.2mg ( 4.58 μmol) amino-dT-C6 (see compound 1 in the following reaction formula, purchased from Glen Research) was reacted at 25°C for 2 hours, desalted, acidified (60% CF3COOH-H20, 25°C, 30min), and analyzed by HPLC ( Purification by high performance liquid chromatography) gave 5.1 mg (92%) of compound 3. The specific reaction formula is as follows:
[0045]
[0046] 2. Phosphoramidation reaction:
[0047] Compound 3 (1.835g, 1.5mmol) was repeatedly rotary evaporated with anhydrous pyridine (1mL×3), dry toluene ((1mL×1) and dry acetonitrile (1mL×1) to obtain dry compound 3, which was dissolved in dry dichloromethane (15mL), bis(diisopropylami...
Embodiment 2
[0050] Example 2, the preparation of DQ (synthesis and purification of MGB modified dA):
[0051] The synthesis and purification of MGB-modified dA and the phosphoramidation reaction can be carried out according to the process of Example 1, using amino-dA-C6 (purchased from Glen Research), other substances and their molar ratios are the same as in Example 1, and the obtained compound 5 The structure is:
[0052]
[0053] The structural data of compound 5 are as follows: 1H NMR (CDC13) δ12.00 (d, 1H), 11.54 (s, 1H), 8.28 (d, 1H), 8.16 (d, 1H), 8.03 (m, 2H), 7.97 (d, 1H), 7.11-7.37(m, 12H), 6.99(m, 2H), 6.94(d, 1H), 6.81(d, 4H,), 6.37(d, 1H,), 6.21(t, 1H ,), 6.01(s, 2H), 4.62(t, 2H), 4.54-4.60(m, 1H), 4.12-4.13(m, 1H), 3.98(t, 2H), 3.75(s, 6H), 3.52 (d, 1H), 3.41 (t, 3H), 3.29 (t, 2H), 2.67-2.77 (m, 1H). m / z (M+H) of 1376.59 was found to be 1477.39 by mass spectrometry.
Embodiment 3
[0054] The preparation of embodiment 3, DQ
[0055] 1. Synthesis and purification of MGB modified dT:
[0056] Dissolve 10.12 mg (12.52 μmol) of DPI3 (see compound 6 in the following reaction formula, purchased from Shanghai Huirui Biotechnology Co., Ltd.) in 8ml DMF (dimethylformamide) and 8mL 0.1M NaHCO3, add 5mg ( 7.16 μmol) amino-dT-C6 (see compound 1 in the following reaction formula, purchased from Glen Research) was reacted at 25°C for 2 hours, desalted, acidified (60% CF3COOH-H2O, 25°C, 30min), and analyzed by HPLC ( Purification by high performance liquid chromatography) yielded 78.96 mg (89%) of the compound. The specific reaction formula is as follows:
[0057]
[0058] 2. Phosphoramidation reaction:
[0059] Compound 7 (2.111g, 1.5mmol) was repeatedly rotary evaporated with anhydrous pyridine (1mL×3), dry toluene ((1mL×1) and dry acetonitrile (1mL×1) to obtain dry compound 7, which was dissolved in dry dichloromethane (15mL), bis(diisopropylamino)(2-cyanoeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com