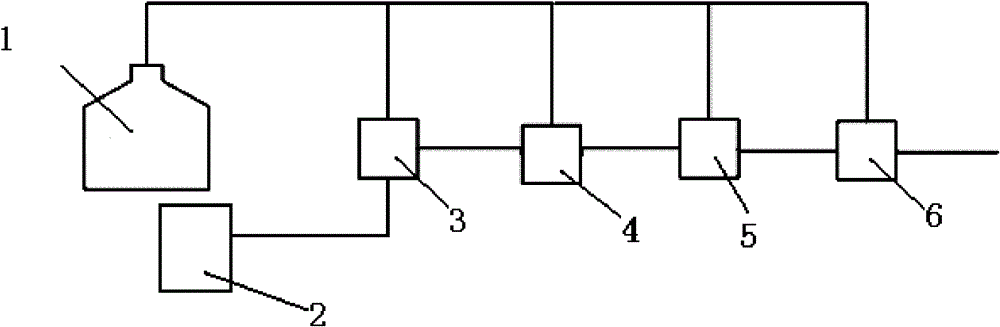
Multistage rearrangement system and method for preparation of caprolactam from cyclohexanone oxime
A technology for cyclohexanone oxime and caprolactam, which is applied in the field of preparing caprolactam from cyclohexanone oxime by multi-stage rearrangement, can solve the problems of difficult temperature control, low production capacity, incomplete reaction and the like, and achieves improved controllability and safety, Strictly control the residence time and improve the effect of reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing caprolactam from cyclohexanone oxime by multi-stage rearrangement is carried out according to the following steps:
[0028] a. Dissolving cyclohexanone oxime in n-octane solution to form a solution with a cyclohexanone oxime mass fraction of 10%, and adding it to the cyclohexanone oxime liquid addition tank;
[0029] B, with SO Content is 20wt.% fuming sulfuric acid as dispersed phase, cyclohexanone oxime solution is as continuous phase, injects first micro-mixer through oleum filling tank and cyclohexanone oxime filling tank respectively, disperses The phase flow rate is 1.1mL / min, the flow rate of the continuous phase injected into the first micro-mixer is 20mL / min, and the Beckmann rearrangement reaction is caused by rapid mixing, and the temperature of the reaction system is 90°C;
[0030] c. Pass the mixture after the Beckmann rearrangement reaction into the second micro-mixer, mix with the cyclohexanone oxime solution added in the second mic...
Embodiment 2
[0034] A method for preparing caprolactam from cyclohexanone oxime by multi-stage rearrangement is carried out according to the following steps:
[0035] a. Dissolving cyclohexanone oxime in n-octane solution to form a solution with a mass fraction of cyclohexanone oxime of 1%, and adding it to the cyclohexanone oxime liquid addition tank;
[0036] b. Put SO 3 Content is the oleum of 20wt.% as dispersed phase, and cyclohexanone oxime solution is used as continuous phase, injects the first micro-mixer through oleum filling tank and cyclohexanone oxime filling tank respectively, and dispersed phase flow rate is 2.4 mL / min, the flow rate of the continuous phase injected into the first micro-mixer is 20mL / min, rapid mixing and triggering Beckmann rearrangement reaction, the temperature of the reaction system is 90°C;
[0037] c. Pass the mixture after the Beckmann rearrangement reaction into the second micro-mixer, mix with the cyclohexanone oxime solution added in the second mic...
Embodiment 3
[0041] A method for preparing caprolactam from cyclohexanone oxime by multi-stage rearrangement is carried out according to the following steps:
[0042] a, dissolving cyclohexanone oxime in n-octane solution, making cyclohexanone oxime mass fraction is 18% solution, joins cyclohexanone oxime filling tank;
[0043] b. Put SO 3 The oleum with a content of 20wt.% is used as the dispersed phase, and the cyclohexanone oxime solution is used as the continuous phase, which is injected into the first micro-mixer through the oleum filling tank and the cyclohexanone oxime filling tank respectively, and the flow rate of the dispersed phase is 2.0 mL / min, the flow rate of the continuous phase injected into the first micro-mixer is 20mL / min, rapid mixing and triggering Beckmann rearrangement reaction, the temperature of the reaction system is 70°C;
[0044] c. Pass the mixture after the Beckmann rearrangement reaction into the second micro-mixer, mix with the cyclohexanone oxime solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com