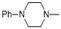Novel pleuromutilin derivate, preparation method and medical use thereof
A technology of pleuromutilin and derivatives, which is used in pharmaceutical formulations, antibacterial drugs, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
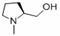
[0092] 14-Deoxy-[(2-(p-toluenesulfonyloxy)acetoxy]-mutriptyline (2) Preparation
[0093] Dissolve 20 g (52.9 mmol) of pleuromutilin in 160 mL of 1,2-dichloroethane, and then add 12 mL (86.6 mmol) of triethylamine to obtain a yellow clear liquid. Add 14 g (73.7 mmol) of p-toluenesulfonyl chloride and react at room temperature (20°C). As the reaction progresses, the solution gradually turns yellow and turbid. After 17 h, the reaction was complete. After stopping the stirring, let it stand still, remove the solid by filtration, wash the filtrate twice with water until pH = 7, and dry it with anhydrous sodium sulfate. The solution was vortexed until dry, and a large amount of white matter was produced. The resulting solid was recrystallized from ethyl acetate, and filtered with suction to obtain 14.2 g of a white solid. Yield: 50.4% Melting point 141-144°C.
[0094] Preparation of deoxy-(2-iodoacetoxy)-mutriptyline (3)
[0095] Dissolve 1.08 g (6.54 mmol) of potassium iodid...
Embodiment 2
[0104] 14-Deoxy-[2-[2-[2-(piperidinyl)acetamido]-thiazole-4-methylthio]acetoxy]-mutriptyline (II 2 ) preparation
[0105] Refer to I I 1 The preparation method, by ( 5 ) reacted with hexahydropyridine, a white foamy solid with a yield of 65%. 1 HNMR (CDCl 3, 400 MHz): δ 10.42 (1H, br, NH), 6.79 (1H, s, H25), 6.51 ( 1H, dd, J 1 =10.8, J 2=17.2, H19), 5.79 (1H, d, J =8.4, C14), 5.39 (1H, d, J =10.8, C20), 5.24 (1H, d, J =17.6, C20), 3.83 (2H, s, H23), 3.38 (1H, m, H11), 3.18 (2H, s, H22), 3.06 (2H, s, H28), 2.52 (4H, br, 2× NCH 2 ), 2.39~2.05 (5H, m, H2, H4, H10, H13), 1.80~1.19 (20H, m, H1, H6, H7, H8, H15, H13,11-OH, H18, 3×CH 2 ), 0.89 (3H, d, J =6.8, H17), 0.75 (3H, d, J =6.8, H16); 13 CNMR (CDCl 3, 400 MHz): δ 217.10 (C3), 168.64 (C21), 157.83 (C27), 146.17 (C26), 139.08 (C24), 117.30 (C19), 111.32 (C20), 107.03 (C25), 74.61 (C11), 69.19 (C14), 61.89 (CH 2 NCH 2 ), 58.17 (C4), 56.68 (C28), 45.44 (C22), 45.38 (C9), 43.89 (C13), 41.72 (C12), 36.78 ...
Embodiment 3
[0107] 14-Deoxy-[2-[2-[2-(morpholinyl)acetamido]-thiazole-4-methylthio]acetoxy]-mutriptyline (II 3 )
[0108] Refer to I I 1 The preparation method, by ( 5 ) reacted with morpholine, white foamy solid, yield 61%. 1 HNMR (CDCl 3, 400 MHz): δ 7.27 (CHCl 3 ), 6.81 (1H, s, H25), 6.50 (1H, dd, J 1 =11.2, J 2 =17.6, H19), 5.79 (1H, d, J =8.4, H14), 5.38 (1H, d, J =11.2, H20), 5.24 (1H, d, J =17.6, H20), 3.83 (2H, s, H23), 3.79 (4H, t, C H 2 OC H 2 , 3.38 (1H, m, H11), 3.27 (2H, s, H22), 3.05 (2H, s, H28), 2.64 (4H, br, CH 2 NCH 2 ), 2.39~2.05 (5H, m, H2, H4, H10, H13), 1.80~1.14 (16H, m, H1, H6, H7, H8, H15, H13, 11-OH, H18), 0.89 (3H, d, J =7.2, H17), 0.75 (3H, d, J =6.8, H16); 13 CNMR (CDCl 3, 400 MHz): δ 217.06 (C3), 168.61 (C21), 157.68 (C27), 146.27 (C26), 139.13 (C24), 117.25 (C19), 111.46 (C20), 107.04 (C25), 74.62 (C11), 69.28 (C14), 61.00 (CH 2 OCH 2 ), 59.18 (C4), 57.63 (C28), 53.52 (CH 2 NCH 2 ), 45.46 (C22), 44.84 (C9), 43.92 (C13), 41.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com