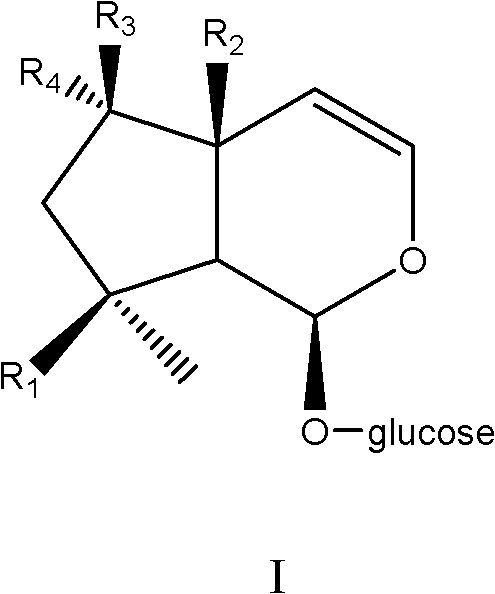
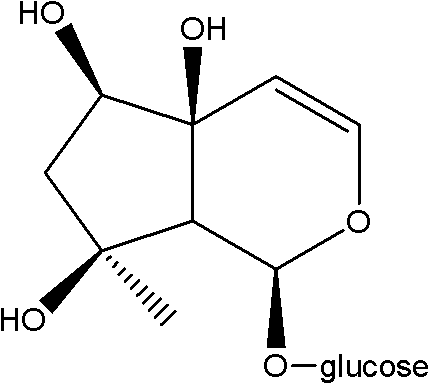
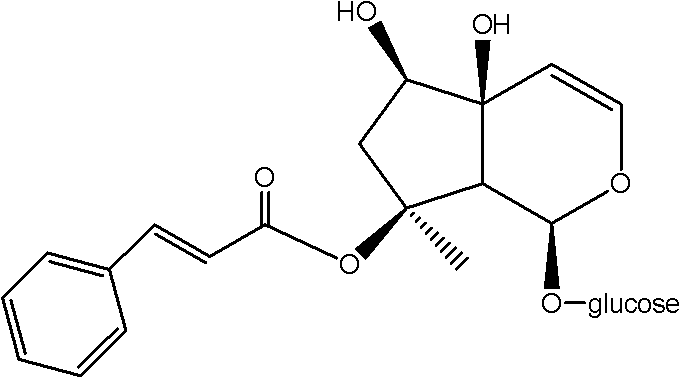
Medical application of Harpagoside ingredient
A technology of use and pharmacy, which is applied in the field of medical use of harpagoside compounds, and can solve unseen problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Hypoglycemic experiment in normal mice
[0029] experimental method:
[0030] 90 healthy mice were randomly divided into 9 groups according to body weight, 10 in each group. The 7 groups of administration groups were given harpagoside 5mg / kg, harpagoside 10mg / kg, harpagoside 5mg / kg, harpagoside 10mg / kg, 8-cinnamoyl predjoside 5mg / kg, 8-O-trans Formula-p-hydroxycinnamoyl harpaside 5mg / kg, 8-O-trans-p-hydroxycinnamoyl harpaside 10mg / kg, the control group was given the same amount of normal saline, and the positive drug group was given metformin 140mg / kg, administered by intragastric administration , once a day, for 7 days in a row. Fasting for 2 hours before the last administration (without water), and measuring fasting blood glucose (FBG) 1 hour after administration.
[0031] Experimental results:
[0032] Table 1 hypoglycemic effect on normal mice ( n=10)
[0033]
[0034] * Indicates that the administration group and the positive drug group are significantly ...
Embodiment 2
[0037] Hypoglycemic experiment in acute insulin resistant mice:
[0038] experimental method:
[0039] 1. Prepare the supernatant of activated macrophages. Experiments were carried out after mice were adaptively fed for one week (body weight was about 25 g). Mice were killed by dislocation, macrophages were prepared, stimulated with LPS (2 μg / ml), and the supernatant was collected 48 hours later. TNF-a and IL-6 were measured by ELISA, and the contents of the two inflammatory cytokines were significantly higher than those in the control group, indicating that the stimulation was successful, and they were stored at -70°C for later use.
[0040] 2 Preparation of inflammatory insulin resistance model. The mice were fasted for 12 hours, administered by intragastric administration, and 30 minutes later, 200 μl of the supernatant of activated macrophages was injected intraperitoneally (diluted according to a certain ratio). After 1 hour, glucose (4 g / kg) was given by intragastric...
Embodiment 3
[0048] 3T3-L1 adipocyte sugar uptake experiment
[0049] experimental method:
[0050] a) 3T3-L1 preadipocyte culture
[0051] 3T3-L1 preadipocytes were placed in DMEM medium (containing 0.1 g / L streptomycin and 100 U / ml penicillin) with 10% neonatal calf serum at 37°C, 5% CO 2 , cultured under saturated humidity conditions. Cells in the logarithmic growth phase were used for experiments.
[0052] b) Effect on the viability of 3T3-L1 preadipocytes
[0053] Take 4×10 4 Cells / ml 180μl were seeded in 96-well plate, 37℃, 5%CO 2 , cultured under saturated humidity conditions. When the cell density reached about 85%, different concentrations of the test drug were added for 48 hours. The supernatant was discarded, and 180 μl of serum-free DMEM and 20 μl of MTT (5 mg / ml) were added to each well to continue culturing at 37° C. for 4 h. Then carefully discard the supernatant, add 150 μl DMSO to each well and shake for 10 min, measure the absorbance at 570 nm, and calculate the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com