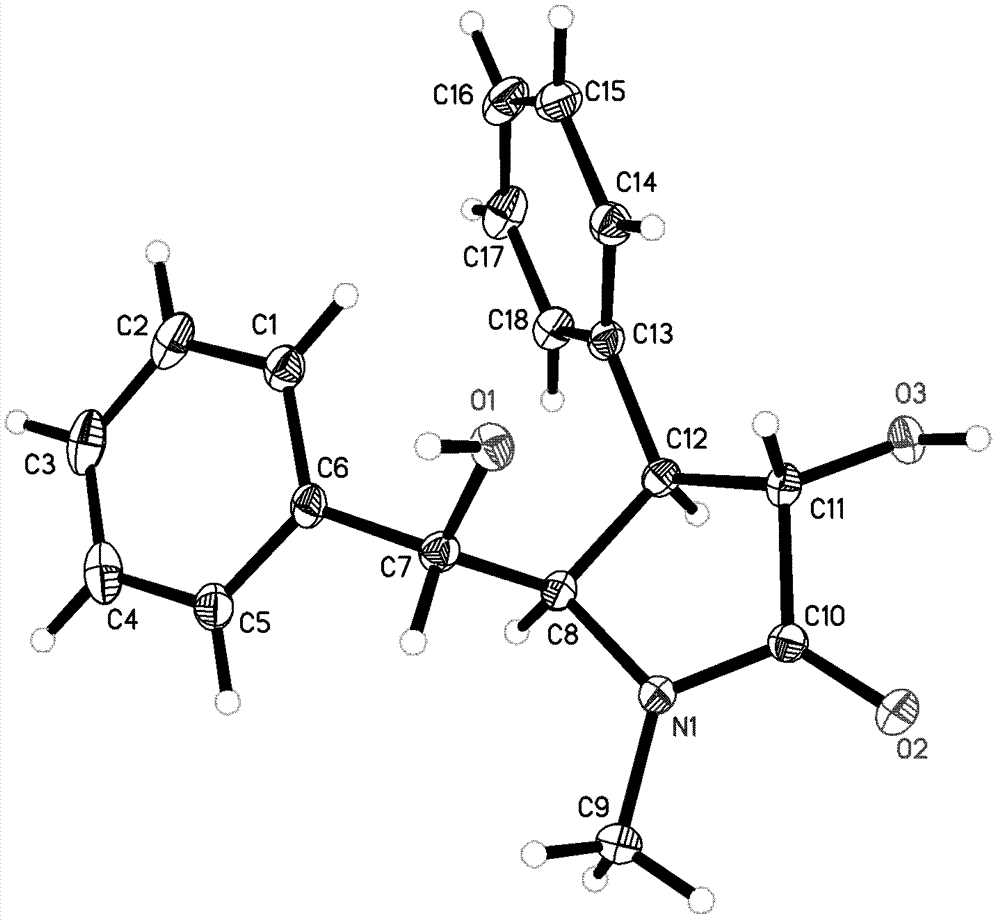
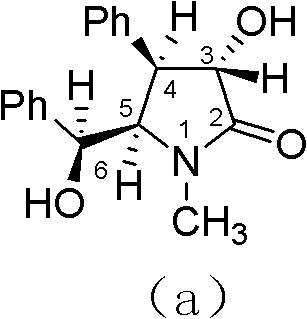
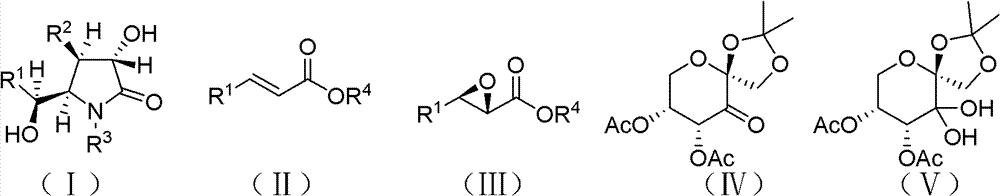
Preparation method of optically pure (-)-clausenamide compound
A technique for the production of pancetamide and its compounds, which is applied in the field of preparation of -bantamide compounds, can solve the problems of restricting the industrial production of optically pure (-)-bantamide, and achieve the environmental protection of the oxidation method, the improvement of efficiency, and the post-treatment easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1, (-)-bantamide
[0040] (1) Preparation of (+)-(2S, 3R)-t-butyl epoxy cinnamate
[0041]
[0042] The reaction equation is shown in the above formula, where Ph is phenyl; t-Bu is tert-butyl; Ac is acetyl;
[0043] Add trans-tert-butyl cinnamate (1.0mol, 204.0g) dissolved in 5.0L acetonitrile to a 50L reaction kettle with a mechanical stirrer cleaned with deionized water, and dissolve in 2.5L acetonitrile to obtain the concentration It is a chiral ketone derived from fructose shown in formula (1) of 0.12M (wherein, the molar fraction ratio of tert-butyl cinnamate and chiral ketone derived from fructose shown in formula (1) is 1: 0.30), and Add tetra-n-butylammonium bisulfate (0.06mol, 20.0g), and then add 5.0L of 1×10 -4 The aqueous solution of disodium ethylenediaminetetraacetic acid of M; pass the cooling liquid into the interlayer of the reaction kettle, adjust the temperature in the reaction kettle to be -5°C; add 3.08kg of the pu...
Embodiment 2
[0063] The preparation of embodiment 2, (-)-bantamide
[0064] (1) Preparation of (+)-(2S, 3R)-epoxycinnamic acid ethyl ester
[0065]
[0066] The reaction equation is shown in the above formula, where Ph is phenyl; Ac is acetyl;
[0067] In the 5L reaction kettle with mechanical stirrer that has been cleaned with deionized water, add the trans ethyl cinnamate (0.1mol, 17.62g) that is dissolved in 0.5L acetonitrile, the concentration obtained in being dissolved in 0.25L acetonitrile is The chiral ketone derived from fructose shown in 0.12M formula (1) (wherein, the molar fraction ratio of ethyl cinnamate and the chiral ketone derived from fructose shown in formula (1) is 1: 0.30), and adding four n-Butylammonium bisulfate (0.006mol, 2.0g), then add 0.5L1×10 -4 Aqueous solution of disodium ethylenediaminetetraacetic acid of M; pass cooling liquid into the interlayer of the reaction kettle, adjust the temperature in the reaction kettle to -5°C-5°C; 0.308kg potassium hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com