A kind of preparation method of hydrogen selenide
A technology of hydrogen selenide and hydrogen, applied in the direction of binary selenium/tellurium compounds, etc., can solve the problems of frozen blockage at the outlet of the transfer pipe, interruption of the product transfer process, high energy consumption, etc., and achieve the effect of reducing energy consumption and avoiding freezing blockage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
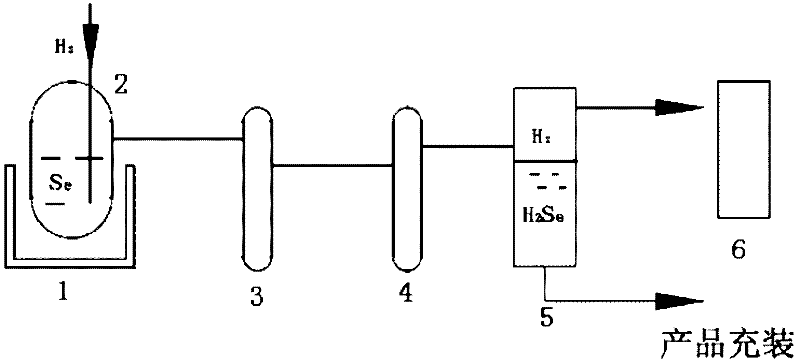
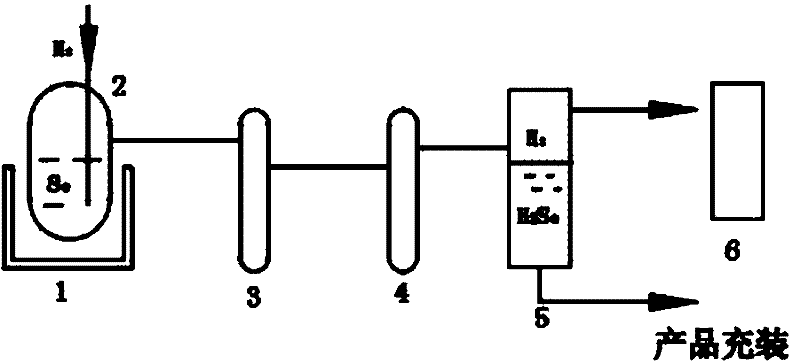
[0036] 1) Add 10 kilograms of selenium particles into the reactor, seal the system, and use helium to carry out air tightness test.
[0037] 2) After passing the airtight test, vacuumize the reactor to ≤6×10 -2 1 hour below Pa.
[0038] 3) After the vacuuming is completed, the reactor is heated to 350° C., and the temperature is kept constant at 350° C. until the reaction is completed.
[0039] 4) Pass hydrogen into the reactor at a flow rate of 5 L / min, and the reaction starts until the liquid level of selenium shown by the radioactive liquid level meter returns to zero, and the reaction ends.
[0040] 5) The hydrogen selenide gas produced by the reaction and the unreacted hydrogen gas mixture pass through the room temperature circulating water heat exchanger and the -40~-60°C low temperature condenser in sequence.
[0041] 6) The hydrogen selenide liquid condensed at low temperature flows into the product collector, the hydrogen tail gas enters the tail gas absorption towe...
example 2
[0044] 1) Add 15 kg of selenium particles into the reactor 2, seal the system, and use helium to carry out air tightness test.
[0045] 2) After passing the airtight test, vacuumize the reactor 2 to ≤6×10 -2 1 hour below Pa.
[0046] 3) After the vacuuming is completed, the reactor 2 is heated to 350° C., and the temperature is kept constant at 350° C. until the reaction is completed.
[0047] 4) Pass hydrogen into the reactor 2 at a flow rate of 7 L / min, and the reaction starts until the liquid level of selenium shown by the radioactive liquid level gauge returns to zero, and the reaction ends.
[0048] 5) The hydrogen selenide gas produced by the reaction and the unreacted hydrogen gas mixture pass through the room temperature circulating water heat exchanger 3 and the -40~-60°C low temperature condenser 4 in sequence.
[0049] 6) The hydrogen selenide liquid condensed at low temperature flows into the product collector 5, and the hydrogen tail gas enters the tail gas abso...
example 3
[0052] 1) Add 30 kg of selenium particles into the reactor 2, seal the system, and use helium to carry out air tightness test.
[0053] 2) After passing the airtight test, vacuumize the reactor to ≤6×10 -2 1 hour below Pa.
[0054] 3) After the vacuuming is completed, the reactor 2 is heated to 500° C., and the temperature is kept constant at 500° C. until the reaction is completed.
[0055]4) Pass hydrogen into the reactor 2 at a flow rate of 9 L / min, and the reaction starts until the liquid level of selenium shown by the radioactive liquid level meter returns to zero, and the reaction ends.
[0056] 5) The hydrogen selenide gas produced by the reaction and the unreacted hydrogen gas mixture pass through the room temperature circulating water heat exchanger 3 and the -40~-60°C low temperature condenser 4 in sequence.
[0057] 6) The hydrogen selenide liquid condensed at low temperature flows into the product collector 5, and the hydrogen tail gas enters the tail gas absorpt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com