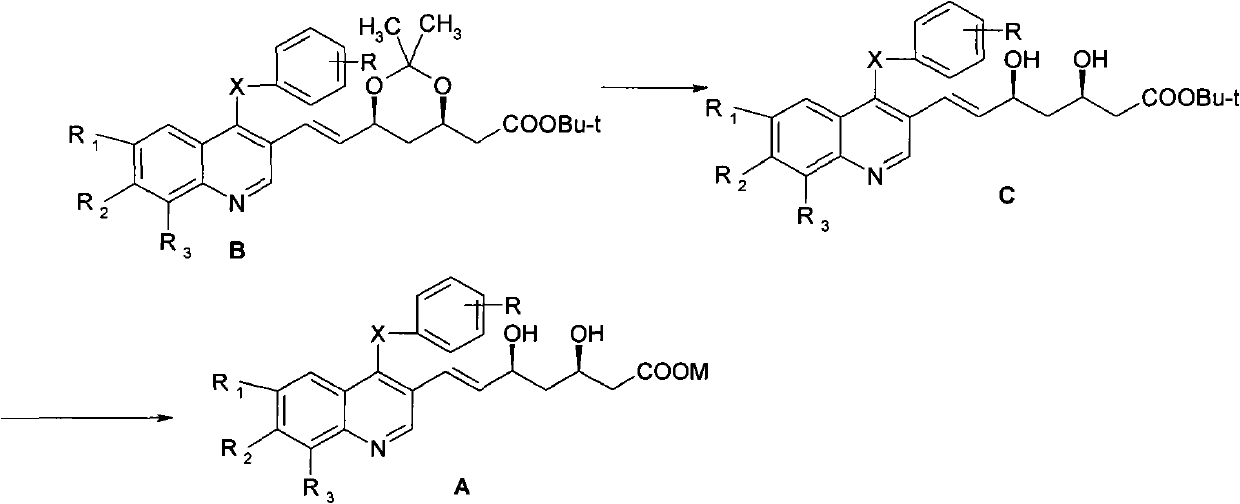
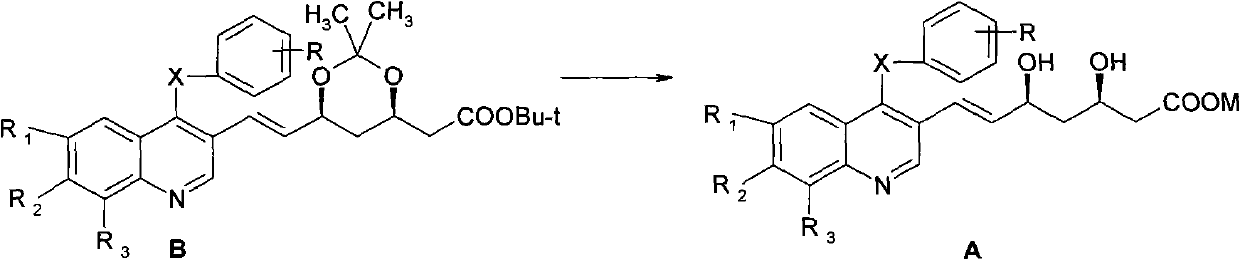
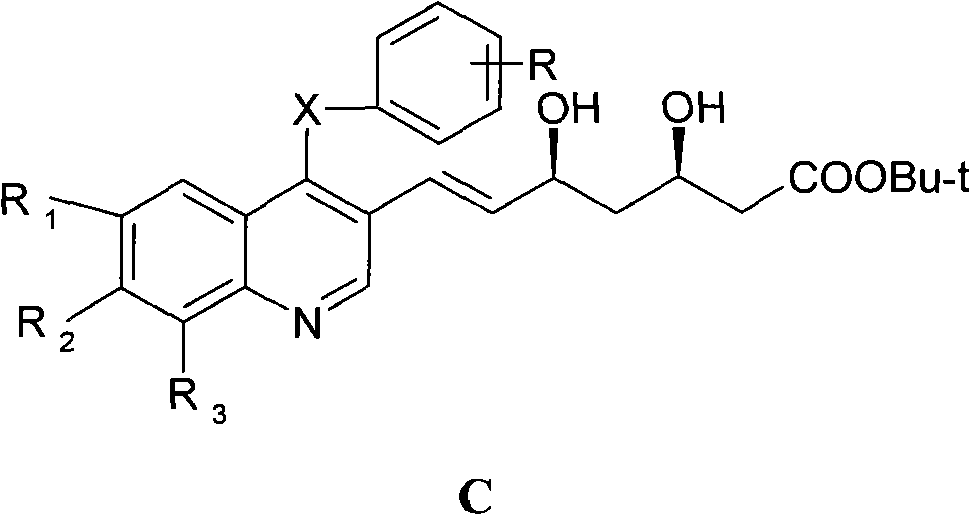
A kind of preparation method of quinoline compound and intermediate compound
A compound and quinoline technology are applied in the field of preparation of quinoline compounds and intermediate compounds, can solve the problems of complicated post-processing, complicated operation and high cost, and achieve the effects of low cost, high total yield and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: (3R,5S)-7-[6,7,8-trifluoro-4-(p-isopropylphenylthio)quinolin-3-yl]-3,5-dihydroxy-6 (E)-Heptenoic acid sodium salt
[0049] (3R,5S)-7-[6,7,8-trifluoro-4-(p-isopropylphenylthio)quinolin-3-yl]-3,5-dihydroxy-3,5-O - Isopropylidene-6(E)-tert-butyl heptenoate 0.1g (0.1mmol) with 1ml ethanol, 1ml THF, cool to 0°C, add 6N HCl 0.1ml (0.6mmol), stir at room temperature for 24h, 0°C Add 0.11ml (1.1mmol) of 10N NaOH, stir for 1h, precipitate out solid, filter with suction, wash with water, wash with ethanol, and dry to obtain 0.04g of yellow solid, yield 44.3%, Mp: 118-119°C, [α] D 26 = 18.2 (c 1 , THF). 1 HNMR (400MHz, DMSO-d 6 )δ1.64-1.10 (m, 6H), 1.68-1.61 (m, 2H), 2.43-2.26 (m, 2H), 2.84-2.71 (m, 1H), 4.06-4.02 (m, 1H), 4.41- 4.37(m, 1H), 6.77(dd, 1H, J=16.4, 5.2Hz), 7.16-7.05(m, 4H), 7.31(dd, 1H, J=16.4, 4.0Hz), 8.12-8.07(m, 1H), 9.32(s, 1H). TOF MS(ES+): 1027(2M+H), 514(M+H).
Embodiment 2
[0050] Example 2: (3R,5S)-7-[6-fluoro-7-chloro-4-p-fluorophenoxy-quinoline-3-]3,5-dihydroxy-6E-heptenoic acid tert-butyl ester
[0051] (3R,5S)-7-[6-fluoro-4,7-diphenylthio-quinoline-3-]3,5-dihydroxy-3,5-O-isopropylidene-6E-heptene Mix and stir 14.2 g (13.0 mmol) of tert-butyl ester and 140 ml of THF, add 5 g (78.0 mmol) of acetic acid at 0°C, stir at room temperature at 25°C for 12 hours, filter the solid with suction, dissolve the solid in 100 mL of ethyl acetate and 70 mL of water, and wash with water until Neutral, anhydrous Na 2 SO 4 Dry, concentrate, and dry to obtain 5.6g of solid, yield 42.7%, mp: 136-138°C, [α] D 26 = 27.8 (c 1, methanol). 1 HNMR (400MHz, DMSO-d 6 )δ1.45(s, 9H), 2.81-1.45(m, 2H), 2.37-2.35(m, 2H), 4.15-4.12(m, 1H), 4.68-4.63(m, 1H), 5.77(dd, 1H, J=16.4, 5.1Hz), 6.36(d, 1H, J=16.4Hz), 6.77-6.74(m, 2H), 6.98-6.94(m, 2H), 7.62(d, 1H, J=9.6Hz) 8.21(d, 1H, J=7.2Hz), 8.97(s, 1H).TOF MS(ES+): 1013(2M+H), 506(M+1)
Embodiment 3
[0052] Example 3: (3R,5S)-7-[6-fluoro-7chloro-4-p-fluorophenoxy-quinoline-3-]3,5-dihydroxy-6E-heptenoic acid hemicalcium salt
[0053] 0°C, 5.6ml (56mmol) of 10N NaOH was added dropwise to (3R,5S)-7-[6-fluoro-7-chloro-4-p-fluorophenoxy-quinoline-3-]3,5-dihydroxy - In a solution of 18.8g (37.2mmol) of tert-butyl 6(E)-heptenoate, 190ml ethanol and 190ml THF, stir for 1h, adjust the pH value to 7-8 with 1N HCl at 0°C, and distill off the solvent under reduced pressure. Add 400ml of water and stir to dissolve, add 4.1g (37.2mmol) calcium chloride aqueous solution, stir overnight, precipitate solid, filter with suction, wash with water, recrystallize the obtained solid with 190ml of THF: water (1:1), and place the obtained solid in a vacuum Dry in a drying oven for 24 hours to obtain 14.0 g of off-white solid, yield: 80.0%, mp decomposed at 160°C, HPLC: 99.4%, [α] D 26 =-22.1(c 1,THF:H 2 O=2:1). 1 HNMR (400MHz, DMSO-d 6 )δ1.62~1.54(m, 2H), 2.37-2.20(m, 2H), 3.96-3.92(m, 1H), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com