A green method for the synthesis of α-aminophosphonates catalyzed by brφnsted acidic ionic liquids
An acidic ionic liquid, aminophosphonate technology, applied in chemical instruments and methods, physical/chemical process catalysts, compounds of elements of Group 5/15 of the periodic table, etc., can solve the problem of low reaction yield of electron-withdrawing groups, Problems such as high reaction temperature and long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Acidic ionic liquid [PSb(2-CF 3 Bim)][HSO 4 ] Catalytic synthesis of O, O'-dimethyl-[α-(phenyl)-α-(anilino)]methyl phosphate
[0017] With 2mmol benzaldehyde, 2mmol aniline, 2.2mmol trimethyl phosphite, catalyst 5mol% [PSb (2-CF 3 Bim)][HSO 4 ] was added to a 10mL round-bottomed flask, electromagnetically stirred at room temperature and reacted for 20min until the mixture was solidified, an appropriate amount of distilled water was added to the mixture, the product was stirred and washed with a glass rod, filtered, and the resulting solid was vacuum-dried without recrystallization to obtain the pure product O. O'-Dimethyl-[α-(phenyl)-α-(anilino)]methyl phosphate. Yield 89%. Melting point: 87-90°C.
Embodiment 2
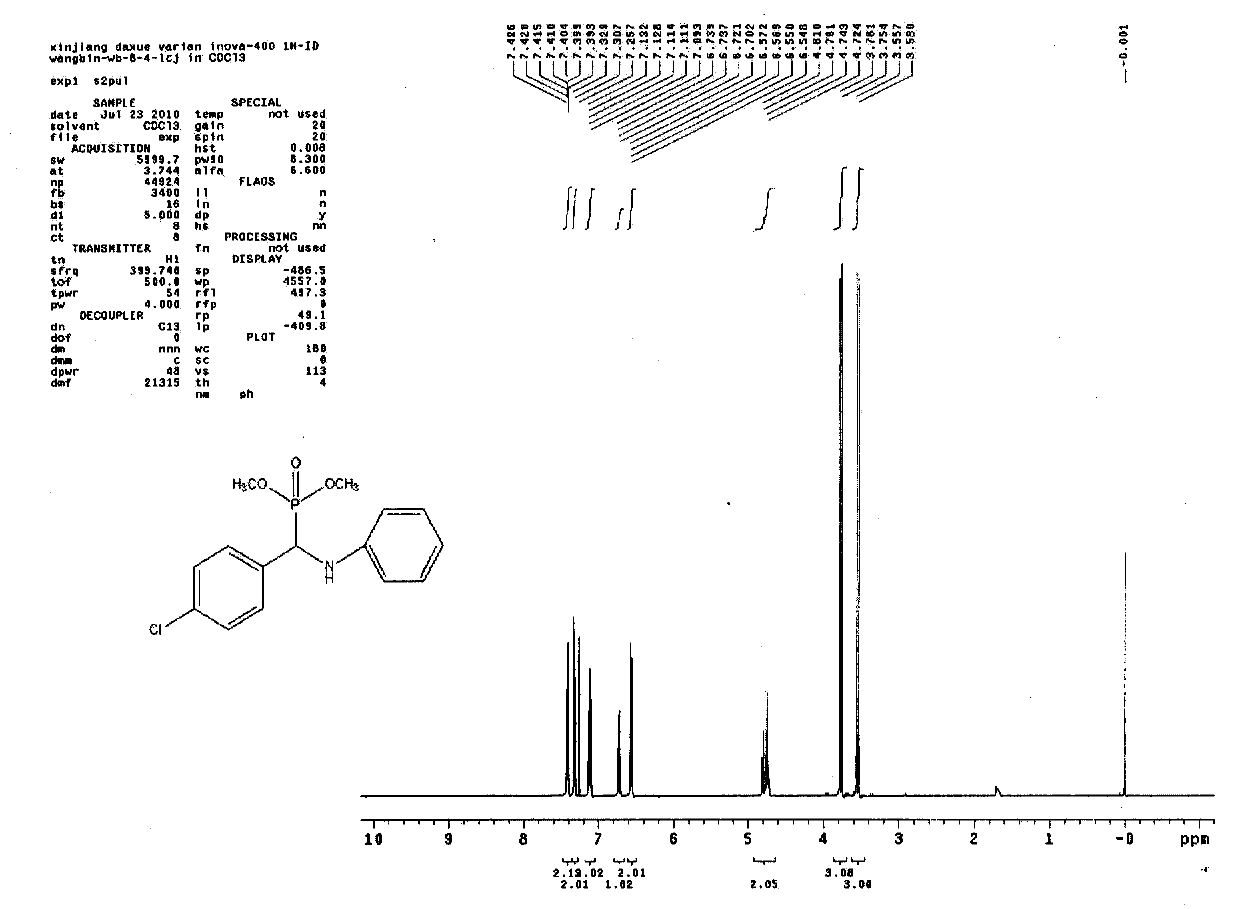
[0018] Example 2: Acidic ionic liquid [PSb(2-CF 3 Bim)][HSO 4 ] Catalytic synthesis of O, O'-dimethyl-[α-(4-chlorophenyl)-α-(anilino)]methyl phosphate
[0019] With 2mmol 4-chlorobenzaldehyde, 2mmol aniline, 2.4mmol trimethyl phosphite, catalyst 5mol% [PSb(2-CF 3 Bim)][HSO 4 ] was added to a 10mL round-bottomed flask, electromagnetically stirred at room temperature and reacted for 20min until the mixture was solidified, an appropriate amount of distilled water was added to the mixture, the product was stirred and washed with a glass rod, filtered, and the resulting solid was vacuum-dried without recrystallization to obtain the pure product O. O'-dimethyl-[α-(4-chlorophenyl)-α-(anilino)]methyl phosphate. Yield 87%. Melting point: 80-82°C.
Embodiment 3
[0020] Example 3: Acidic ionic liquid [PSb(2-CF 3 Bim)][HSO 4 ] catalytic synthesis of O, O'-dimethyl-[α-(4-methoxyphenyl)-α-(anilino)]methyl phosphate
[0021] With 2mmol 4-methoxybenzaldehyde, 2mmol aniline, 2.2mmol trimethyl phosphite, catalyst 5mol% [PSb(2-CF 3 Bim)][HSO 4 ] was added to a 10mL round-bottomed flask, electromagnetically stirred at room temperature and reacted for 15min until the mixture was solidified, an appropriate amount of distilled water was added to the mixture, the product was stirred and washed with a glass rod, filtered, and the resulting solid was vacuum-dried without recrystallization to obtain the pure product O. O'-dimethyl-[α-(4-methoxyphenyl)-α-(anilino)]methyl phosphate. Yield 85%. Melting point: 126-129°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com