Controlled-release composition for producing sustained-release preparation containing udenafil
A technology of Udenafil and sustained-release preparations, applied in the field of controlled-release compositions, to achieve the effects of reducing drug-related side effects, improving particle stickiness, and reducing deviations between individuals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
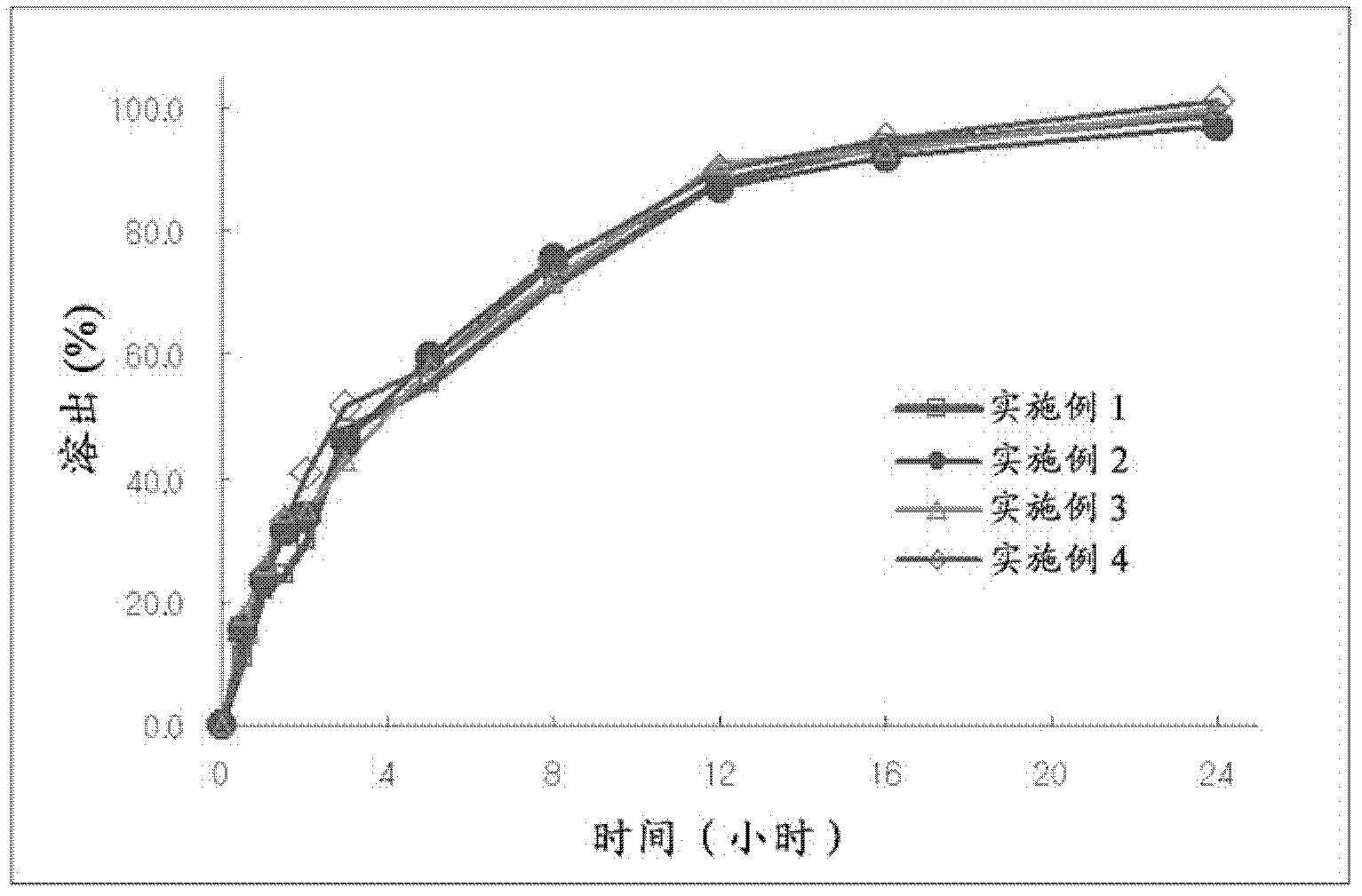
Embodiment 1~4
[0050] The present invention is used to prepare the controlled-release composition containing udenafil sustained-release preparation and its preparation Preparation of the agent
[0051] 1) Manufacture of adsorbent particles for the preparation of controlled release compositions
[0052] Using the components of Examples 1 to 4 described in Table 1 below, adsorbent particles for the production of udenafil controlled-release compositions were produced.
[0053] Specifically, after dissolving Udenafil and citric acid with an appropriate amount of water, the silicon dioxide was then put into a high-speed mixer and the previously prepared Udenafil-citric acid solution was slowly dropped in, the ingredients Adsorbed evenly on the silica surface. The adsorbed particles are put into a high-speed dryer, dried at a temperature of 60 degrees for 30 minutes, and then pulverized.
[0054] 【Table 1】
[0055] Unit: g
[0056]
[0057] 2) Preparation of controlled release composit...
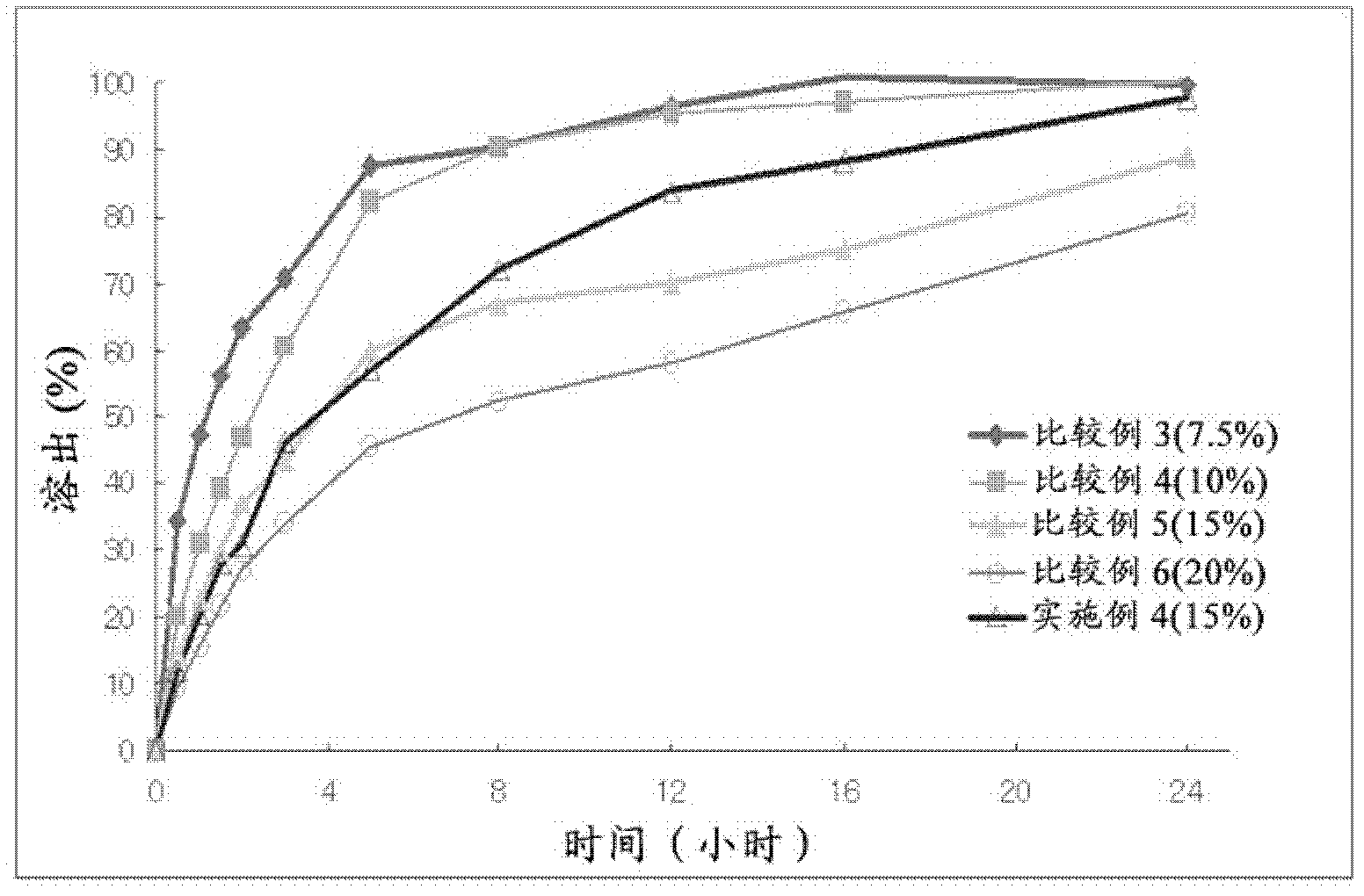
Embodiment 5-12
[0063] The present invention uses various polymers for the preparation of sustained-release formulations containing Udenafil Controlled-release compositions and preparations of formulations
[0064] After the adsorption particles were prepared according to the method of 1) of Example 1 to Example 4, Udenafil was prepared in the same manner as 2) and 3) of Example 1 to Example 4 according to the composition described in Table 3 below. Controlled release formulations and as Examples 5 to 12.
[0065] 【table 3】
[0066]
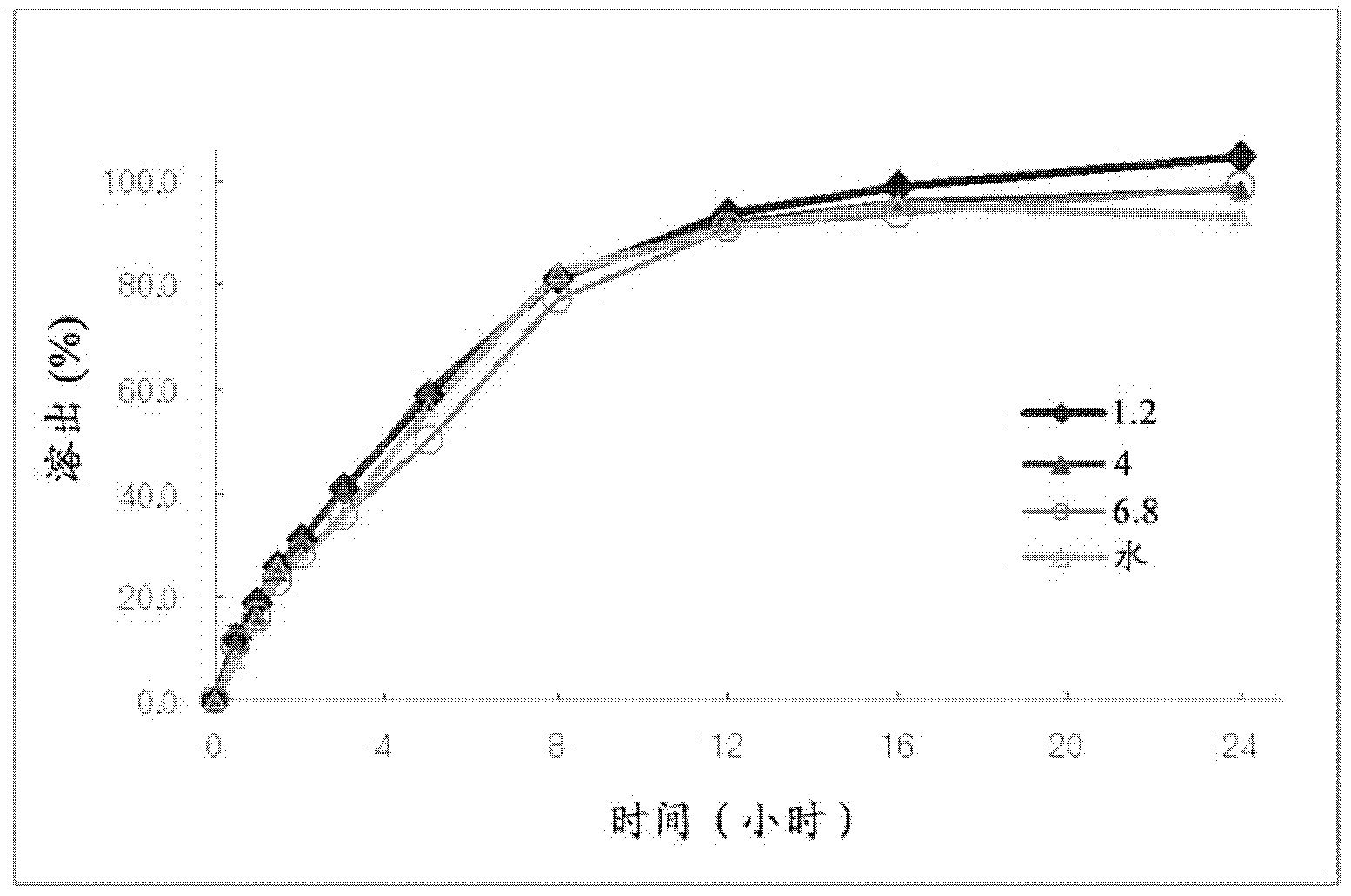
experiment example 1
[0075] Observation of particle fluidity
[0076] In order to confirm whether granules suitable for the preparation of controlled-release preparations could be formed, the granulation degree of the granules produced in 1) of Examples 1 to 4 and the granules produced in Comparative Examples 1 and 2 was visually evaluated using Pharmatest Co., Ltd. The PTG-S3 measures the time for 100 ml of particles to pass through a hole of a certain size and evaluates the particle flowability after drying.
[0077] As shown in Table 6, in Examples 1 to 4 of the present invention in which the Udenafil-citric acid solution was adsorbed and granulated by silica, the particles were in good shape and had good fluidity after drying; However, when granulation was carried out using a general excipient for granules as in Comparative Examples 1 and 2, it was difficult to granulate because of stickiness, and the fluidity after drying was also poor.
[0078] 【Table 6】
[0079] unit
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com