Reagent and method for detecting homocysteine (HCY)
A technology for homocysteine and reagents, which is applied in the field of homocysteine detection and analysis, can solve the problems of time-consuming analysis procedures, high cost and the like, and achieve the effects of simple detection means, low cost and simple detection process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
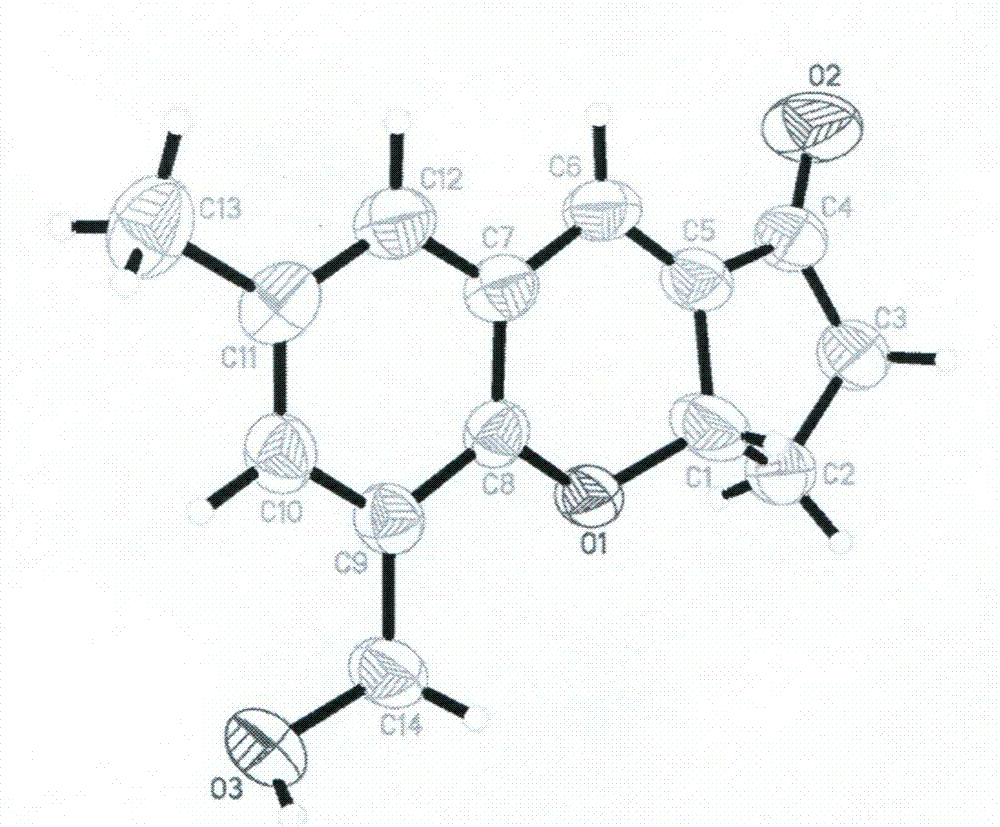
[0024] Synthesis of HMMPC: Mix 2mmoL of 3-hydroxymethyl-5-methylsalicylaldehyde, 3mmoL of cyclopentenone, 3mmoL of imidazole, 3mL of tetrahydrofuran and 3mL of deionized water, stir at room temperature for 48 hours, and dilute with 20mL of 1M HCl , and extracted three times with 30 mL of ethyl acetate, the organic phase was evaporated to dryness under reduced pressure, and then washed with ethyl acetate and petroleum ether in a volume ratio of 1:4 on a silica gel chromatography column to obtain HMMPC with a yield of 56%. The crystal was grown in ethyl acetate and petroleum ether to obtain a light yellow single crystal.
[0025] Characterization of HMMPCs:
[0026] 1 H NMR (600MHz, 25°C, DMSO- d6 ): δ7.18(s, 1H), 7.14(d, 1H), 7.01(s, 1H), 5.30(t, 1H), 2.58-2.63(m, 1H), 2.39-2.74(m, 2H), 2.11-2.38(m, 3H), 2.35(s, 3H); 13 C NMR (150MHz, CDCl 3 ): δ21.23, 28.92, 37.88, 61.94, 76.82, 122.48, 128.61, 129.21, 130.90, 132.36, 133.34, 151.60, 202.06; element analysis (calcd.%) C ...
Embodiment 2
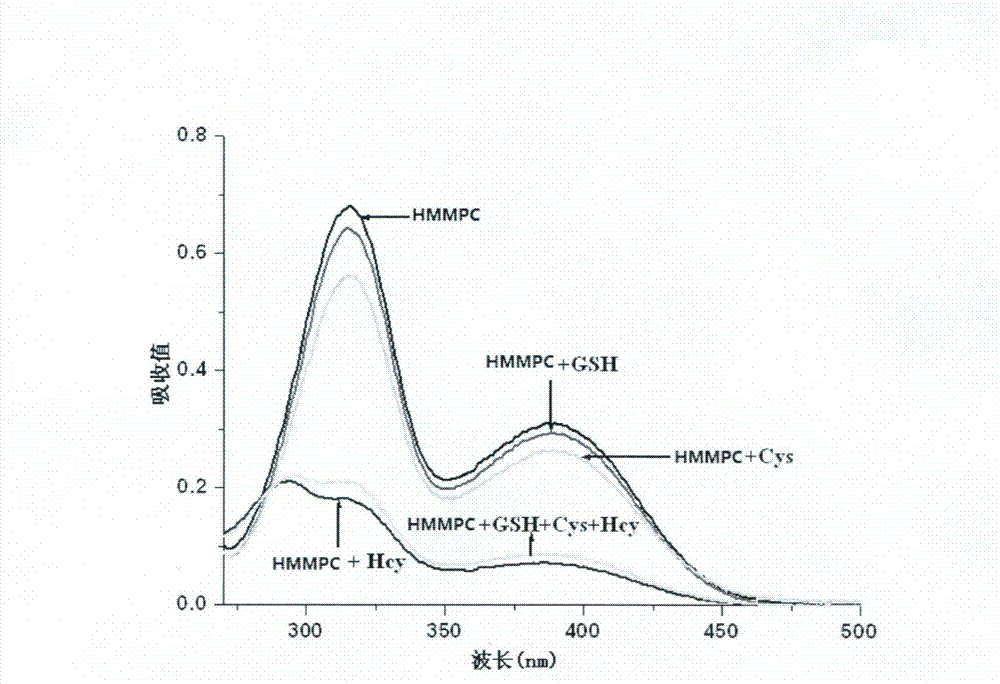
[0028] Add 2ml pH7.0 HEPES (10mM) buffer solution and 2mM, 10μl HMMPC solution to three different UV cuvettes respectively, then add 70μl of Hcy, Cys, GSH respectively, and test on the UV-Vis spectrometer For detection, the UV-visible absorption diagram is shown in figure 2 .
Embodiment 3
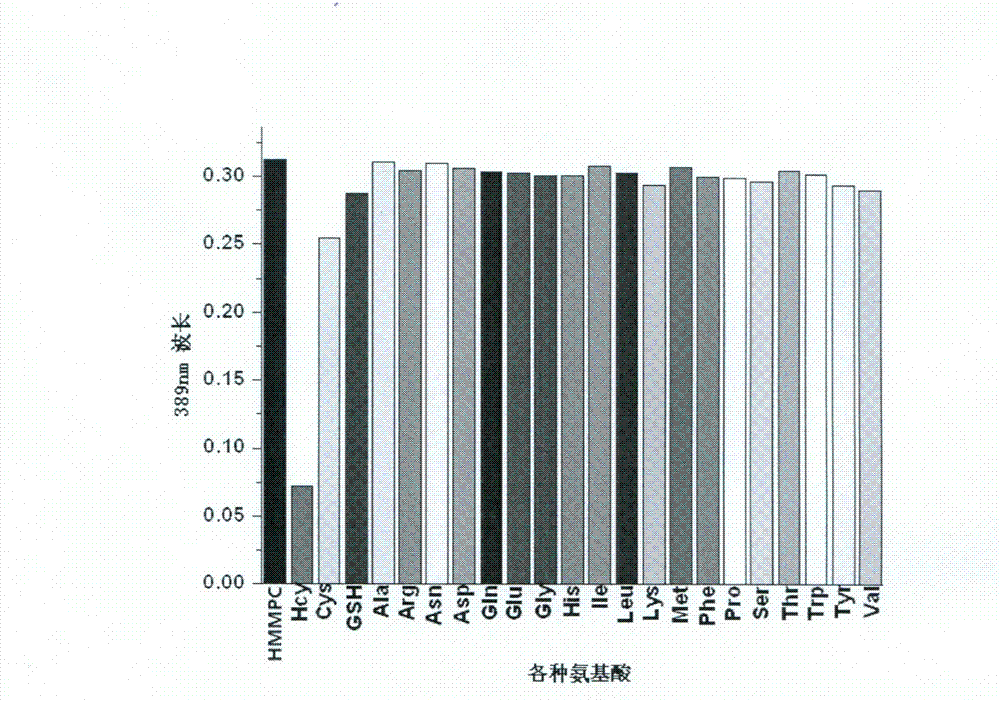
[0030] Add 2ml pH7.0 HEPES (10mM) buffer solution and 2mM, 10μl HMMPC solution to different UV cuvettes respectively, add the same amount of various amino acids respectively, and measure the absorption value at 389nm in a UV-visible spectrometer. To draw a histogram of absorbance values corresponding to different amino acids, see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com