Animal ectoparasite-controlling agent
An ectoparasite, animal body technology, applied in animal repellents, plant growth regulators, biocides, etc., to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0372] Hereinafter, the present invention will be described in detail with reference to preparation examples of the hydrazide compound of the present invention, reference preparation examples of intermediates used for the preparation of the hydrazide compound of the present invention, preparation examples and test examples of the control agent of the present invention, However, the present invention should not be construed as being limited to these examples.
[0373] In this specification, Me represents a methyl group.
[0374] First, the production examples of the hydrazide compound of the present invention are described below:
preparation Embodiment 1
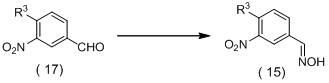
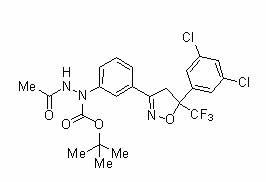
[0376] N'-acetyl-N-{3-[5-(3,5-dichlorophenyl)-5-trifluoromethyl-4,5-dihydroiso Azol-3-yl]phenyl}carbazate (250 mg) was dissolved in tetrahydrofuran (1 mL), and trifluoroacetic acid (1.5 mL) was added dropwise thereto at room temperature, and the mixture was stirred for 1 Hour. To the reaction mixture was added saturated aqueous sodium bicarbonate solution, and the mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue was subjected to silica gel column chromatography to obtain N'-{3-[5-(3,5-dichlorophenyl)-5-trifluoromethyl-4,5-dihydroiso Azol-3-yl]phenyl}acetylhydrazide (179 mg; hereinafter referred to as "hydrazide compound (1) of the present invention").
[0377] Hydrazide compound (1) of the present invention:
[0378]
[0379] Melting point: 100°C
preparation Embodiment 2
[0381] To N'-acetyl-N'-methyl-N-{3-[5-(3,5-dichlorophenyl)-5-trifluoromethyl- 4,5-Dihydroiso To tert-butylazol-3-yl]phenyl}carbazate (324 mg) was added trifluoroacetic acid (5 mL), and the mixture was stirred at this temperature for 30 minutes. The reaction mixture was concentrated under reduced pressure, ethyl acetate was added to the residue, and the organic layer was washed with saturated aqueous sodium bicarbonate. The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The resulting residue was subjected to silica gel column chromatography to obtain N-methyl-N'-{3-[5-(3,5-dichlorophenyl)-5-trifluoromethyl-4,5-dihydroiso Azol-3-yl]phenyl}acetylhydrazide (290 mg; hereinafter referred to as "hydrazide compound (2) of the present invention").
[0382] Hydrazide compound (2) of the present invention:
[0383]
[0384] Melting point: 88°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com