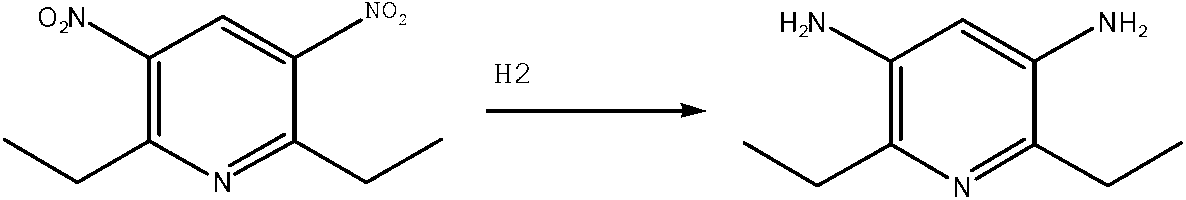
Method for preparing 2,6-dimethoxy-3,5-diamido-pyridine hydrochloride
A technology of diaminopyridine hydrochloride and dimethoxy, applied in the field of preparation 2, can solve the problems of instability, low yield and high cost, and achieve the effects of mild reaction conditions, simple operation and stable content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 2,6-dimethoxy-3,5-dinitropyridine, methanol, water and 5% palladium carbon into the reactor, the weight ratio is 1:0.5:5:0.25; Replace the air with hydrogen, the amount of hydrogen introduced is 0.1~5MPa, heat the reactor until the temperature rises to 55~60°C, stop heating, naturally raise the temperature to 80~85°C, keep for 30min, keep the hydrogen pressure at 3MPa and stop feeding hydrogen , keep warm for 2 hours; after the temperature of the reactor is lowered to below 60°C, pour out the feed liquid, add hydrochloric acid to adjust the pH value to 1, filter and concentrate under reduced pressure; mix the filtrate concentrate with water and activated carbon at a ratio of 1:5:0.1 , decolorized at 60~65°C for 1 hour, then filtered activated carbon, concentrated the filtrate to dryness under reduced pressure; added the resulting filter cake to 1,4-dioxane twice its volume, dispersed and filtered, and dried the filter cake to obtain .
Embodiment 2
[0018] Add 2,6-dimethoxy-3,5-dinitropyridine, methanol, water and 5% palladium carbon into the reactor, the weight ratio is 1:1:1:0.05; Replace the air with hydrogen, and the amount of hydrogen introduced is 0.1~5MPa. Heat the reactor until the temperature rises to 55~60°C, stop heating, naturally raise the temperature to 80~85°C, keep for 40min, and keep the hydrogen pressure at 3MPa to stop feeding hydrogen , keep warm for 3 hours; after the temperature of the reactor is lowered to below 60°C, pour out the feed liquid, add hydrochloric acid to adjust the pH value to 2, filter and concentrate under reduced pressure; mix the filtrate concentrate with water and activated carbon at a ratio of 1:10:0.5 , decolorized at 60~65°C for 2 hours, then filtered activated carbon, concentrated the filtrate to dryness under reduced pressure; added the obtained filter cake to 1,4-dioxane 4 times its volume, dispersed and filtered, and dried the filter cake to obtain .
Embodiment 3
[0020] Add 2,6-dimethoxy-3,5-dinitropyridine, methanol, water and 5% palladium carbon into the reactor, the weight ratio is 1:0.8:4:0.1; Replace the air with hydrogen gas, the amount of hydrogen gas introduced is 0.1~5MPa, heat the reaction vessel until the temperature rises to 55~60°C, stop heating, naturally raise the temperature to 80~85°C, keep it for 30~40min, and keep the hydrogen pressure at 3MPa to stop the flow Add hydrogen and keep warm for 2.5 hours; after reducing the temperature of the reactor to below 60°C, pour out the feed liquid, add hydrochloric acid to adjust the pH value to 1.5, filter and concentrate under reduced pressure; mix the filtrate concentrate with water and activated carbon at a ratio of 1:5 ~10: Mix at 0.1~0.5, decolorize at 60~65℃ for 1~2h, then filter activated carbon, concentrate the filtrate to dryness under reduced pressure; add 3 times the volume of 1,4-dioxane to the obtained filter cake, and disperse Filter and dry the filter cake.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com