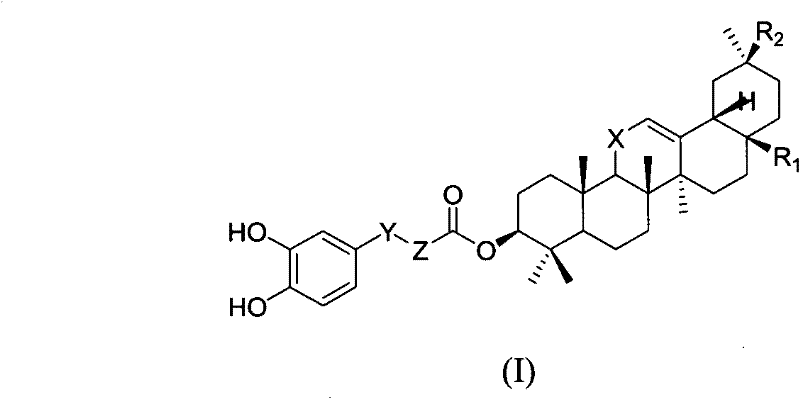
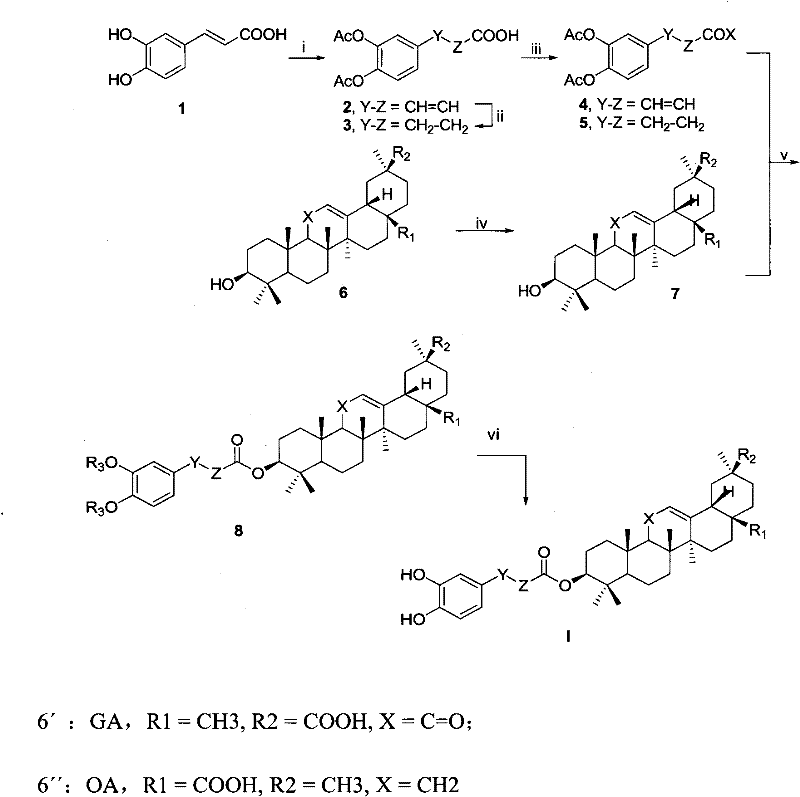
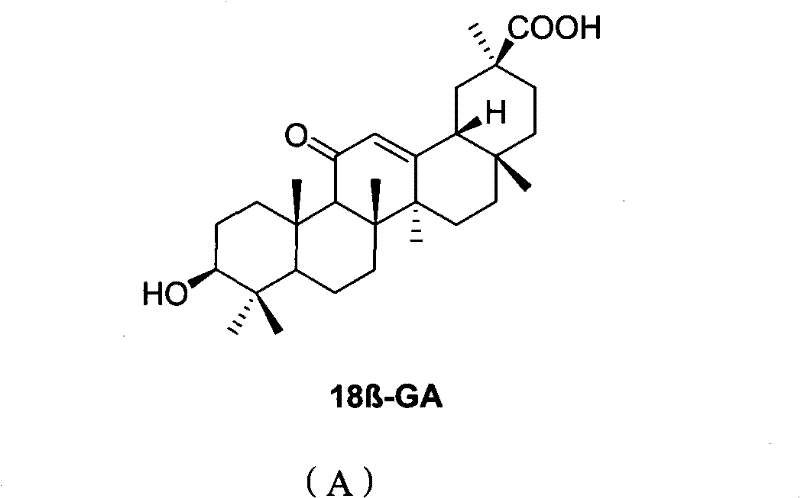
Derivatives of 3-O-caffeoyloleanane type pentacyclic triterpene, preparation method thereof and application thereof
A technology of caffeoyl oleanane and pentacyclic triterpenoids, which is applied in the field of pentacyclic triterpenoid ester derivatives, and can solve problems such as poor absorption, slow dissolution rate, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0066] The preparation of embodiment 1 diacetyl caffeic acid (2)
[0067]
[0068] Dissolve caffeic acid 1 (18.0g, 0.10mol) in 200mL of acetic anhydride, add anhydrous sodium acetate (1.6g, 0.02mol), heat to reflux for 1 hour, cool to room temperature, pour the reaction solution into 2L with vigorous stirring In ice water, stir well for 3 hours. The mixture was suction filtered, the solid was washed three times with water, and dried to obtain diacetylcaffeic acid 2 (23.5 g, yield 89%) as a light yellow solid.
Embodiment 2 2
[0069] The preparation of embodiment 2 dihydrodiacetyl caffeic acid (3)
[0070]
[0071] Diacetylcaffeic acid 2 (2.6g, 10mmol) was dissolved in 50mL of methanol, 5% palladium carbon (Pd-C) catalyst (0.1g) was added, stirred at normal temperature and pressure for 2 hours in a hydrogen atmosphere, and thin-layer chromatography (TLC) detected no starting material. After filtering off Pd-C, the filtrate was desolventized to obtain dihydrodiacetylcaffeic acid 3 (2.5 g, yield 94%) as a white solid.
[0072] Compound 3 1 HNMR (300MHz, CDCl 3 )d: 2.28(s, 3H, O=C-CH 3 ), 2.29 (s, 3H, O=C-CH 3 ), 2.68(t, J=7.8Hz, 2H, C-H 2 ), 2.96(t, J=7.8Hz, 2H, C-H 2 ), 7.04 (s, 1H, Ar-H), 7.10 (s, 2H, Ar-H).
Embodiment 3
[0073] The preparation of embodiment 3 methyl glycyrrhetinate (6a)
[0074]
[0075] Dissolve glycyrrhetinic acid (4.7 g, 10 mmol) in 50 mL of methanol, add 2 mL of concentrated sulfuric acid, and heat to reflux for 3 hours. After cooling to room temperature, pour the reaction solution into 150 mL of water, stir well, extract with dichloromethane (50 mL×3), combine the organic phases, wash with water, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure to obtain white solid glycyrrhetinic acid Methyl ester 6a (4.2 g, 87% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com