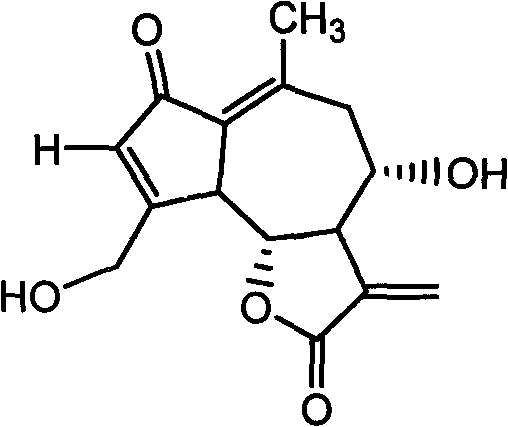
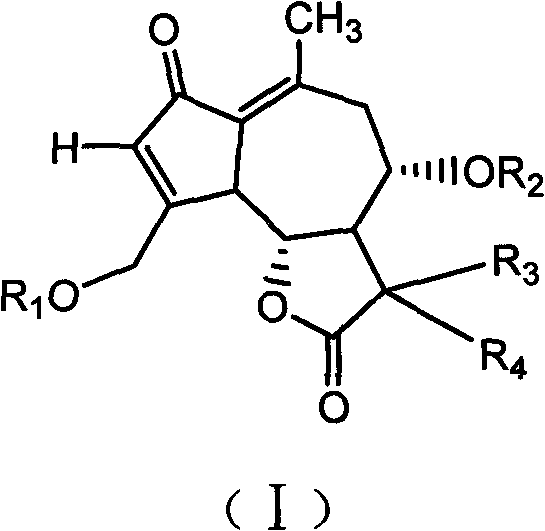
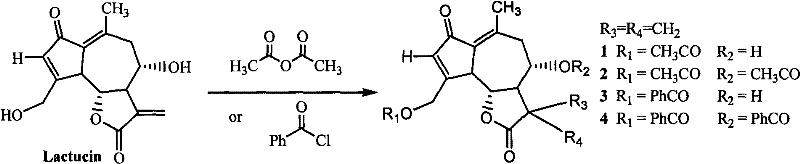
Lactucin derivative, and preparation method and application thereof
A technology of leucanthemum and derivatives, which is applied in the field of natural medicine and medicinal chemistry, can solve the problems of single structure, low toxicity and side effects, and small quantity, and achieves the effects of simple experimental steps and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of derivative 1
[0031] Add 50mg (0.18mmol) lactuca into 1.0ml pyridine, and slowly add 18mg (0.18mmol) acetic anhydride dropwise under cooling in an ice-water bath. After the reaction was completed, the reaction solution was separated by column chromatography to obtain raptudin monoacetate 1.
Embodiment 2
[0032] Embodiment 2: the preparation of derivative 2
[0033] Add 50mg (0.18mmol) lactuca into 1.0ml pyridine, and slowly add 36mg (0.36mmol) acetic anhydride dropwise under cooling in an ice-water bath. After the reaction was completed, the reaction solution was separated by column chromatography to obtain raptudin diacetate 2.
Embodiment 3
[0034] Embodiment 3: the preparation of derivative 3
[0035] Add 50mg (0.18mmol) of Lactuca into 1.0ml of pyridine, and slowly add 25mg (0.18mmol) of benzoyl chloride dropwise under cooling in an ice-water bath. After the reaction was completed, the reaction solution was separated by column chromatography to obtain raptudin monobenzoate 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com