A kind of preparation method of breviscapine crude drug
A technology for breviscapine and raw materials, which is applied in the field of preparation of high-purity breviscapine raw materials, and achieves the effects of simple preparation process, overcoming solvent residues, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
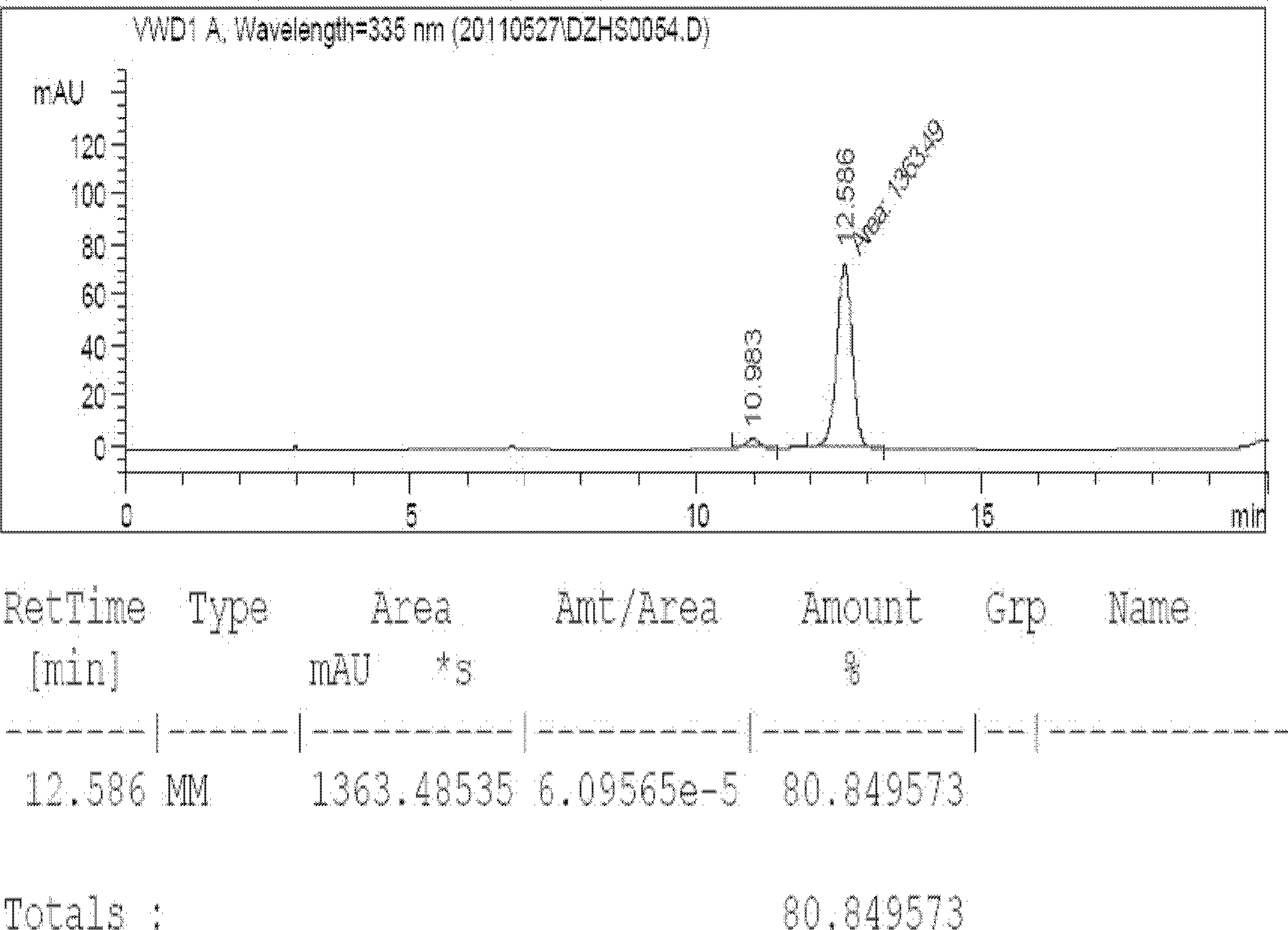
[0020] The raw material of scutellaria breviscapine was extracted with ethanol to obtain crude scutellarin, and the content of scutellarin was detected to be 80.8%. figure 1 For its high-performance liquid chromatography, the detection method and chromatographic conditions are all carried out according to the regulations of the 2010 edition of "Chinese Pharmacopoeia". Weigh 1000g of crude scutellarin, add 35°C warm water to prepare a feed solution with a concentration of 10% by weight, and use 10% NaHCO 3 Adjust the pH to 6.7-7.0, dissolve and filter, centrifuge the filtrate with a 16000r / min continuous tube centrifuge, and then perform fine filtration, adjust the concentration of the filtrate to 10%, heat the temperature to 50°C, adjust the pH to 6.7-7.0, and pack the filtrate Put it into the crystallization tank and let it stand, the total time for natural cooling and crystallization is 60 hours; it takes 38 hours for the temperature of the feed liquid to drop from 50°C to ...
Embodiment 2
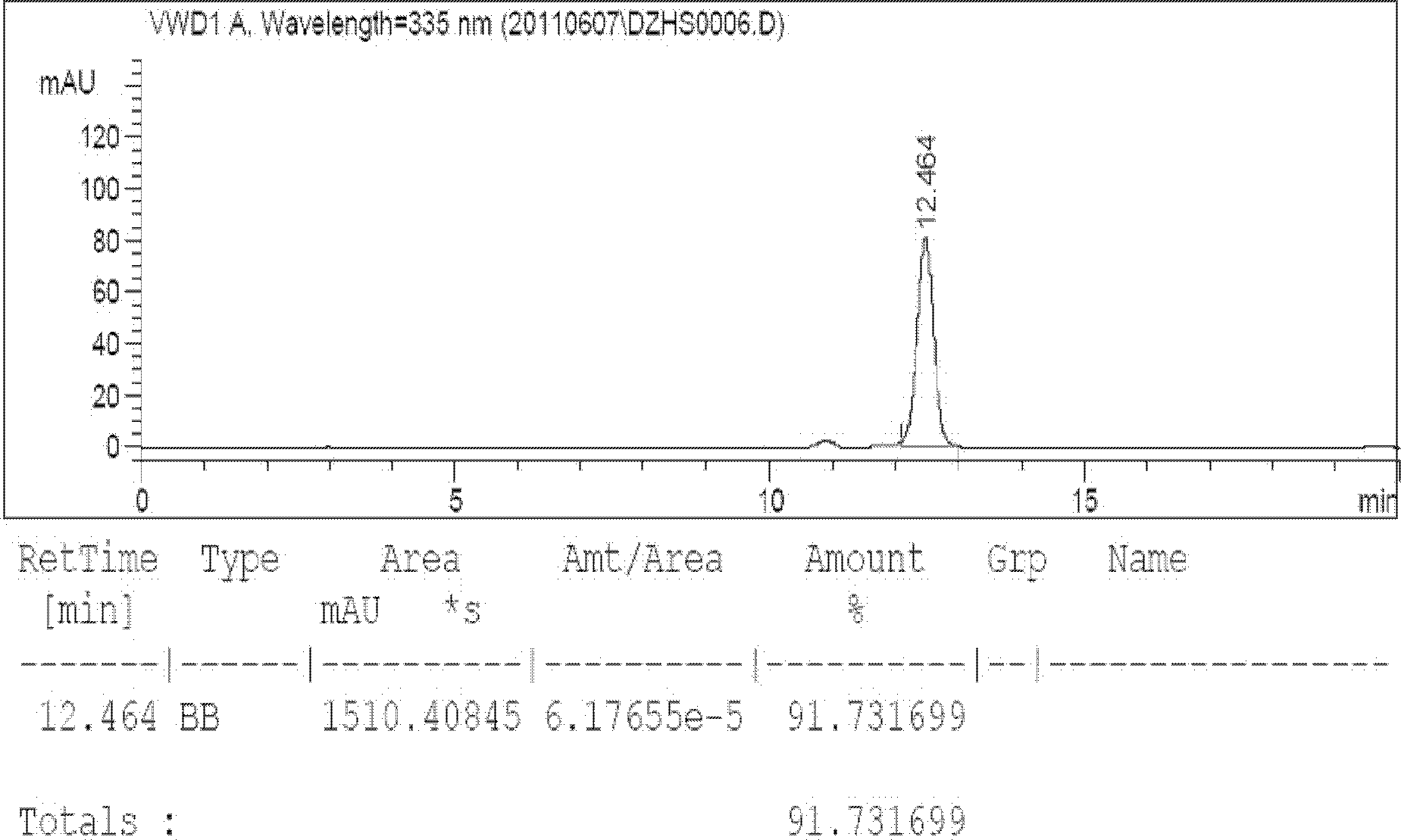
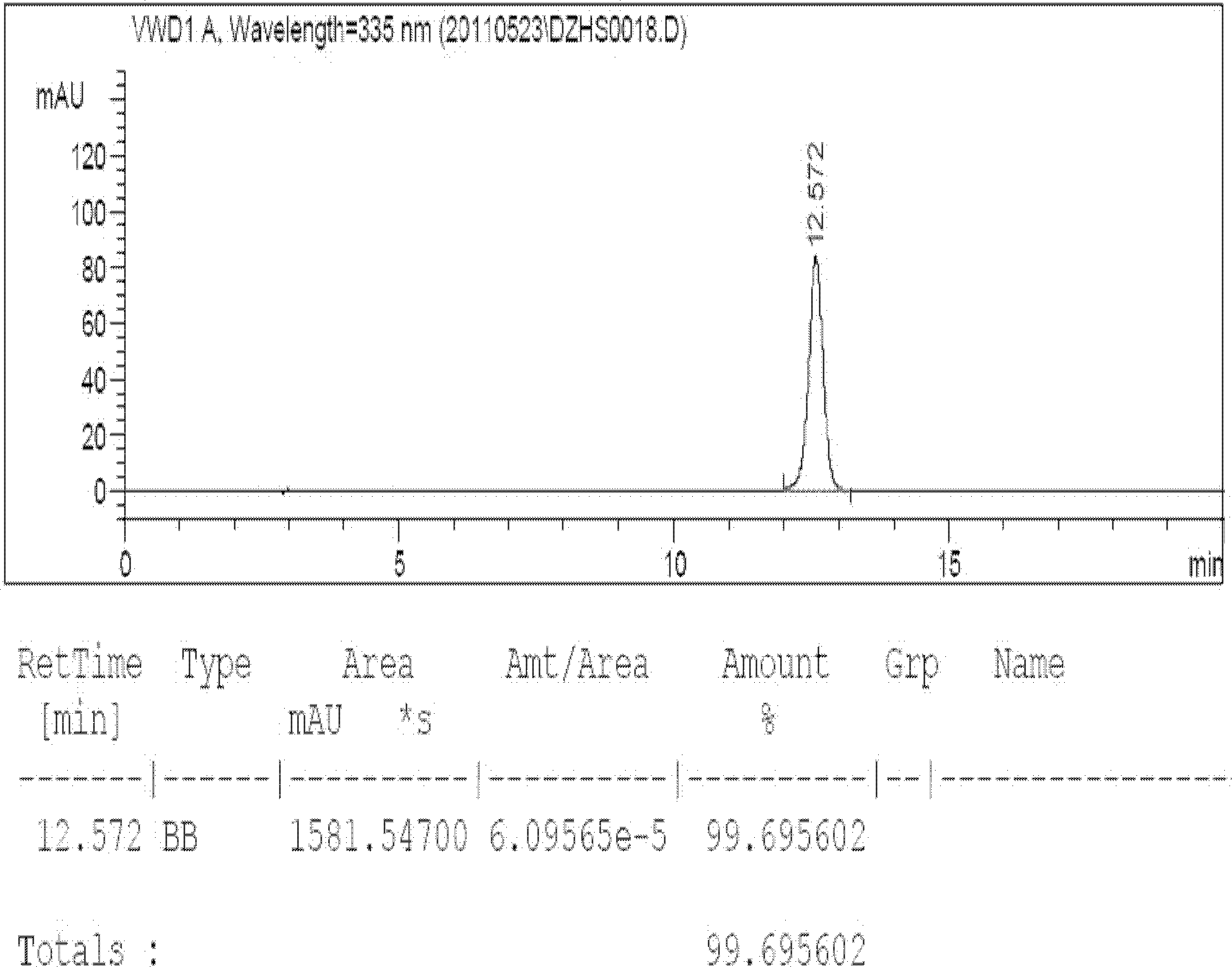
[0022] Take by weighing 1000g of the breviscapine raw material product obtained in Example 1, prepare it into a concentration of 6% by weight, and use 10% NaHCO 3 Adjust the pH to 7.0 to dissolve completely, add activated carbon for needles with a product weight ratio of 3%, heat to 60°C and stir for half an hour to filter while hot, the resulting liquid is clear and transparent, take the filtrate to adjust the concentration to 6.0%, and heat to 80-82 ℃, after adjusting the pH to 6.7-7.0, set the crystallization tank to stand still for 60 hours to crystallize, and cool and crystallize for 48 hours in two stages. The temperature of the filtrate is dropped from 60°C to 46°C. Higher than 0.5°C; the second stage is from 45°C to 22°C, which takes a total of 20 hours, the temperature drop is not higher than 1.15°C per hour, and the crystal is grown for 12 hours; The temperature drops to 22°C, the rotation speed in the tank is 3min / 360°, the crystallization tank and the discharge sys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com