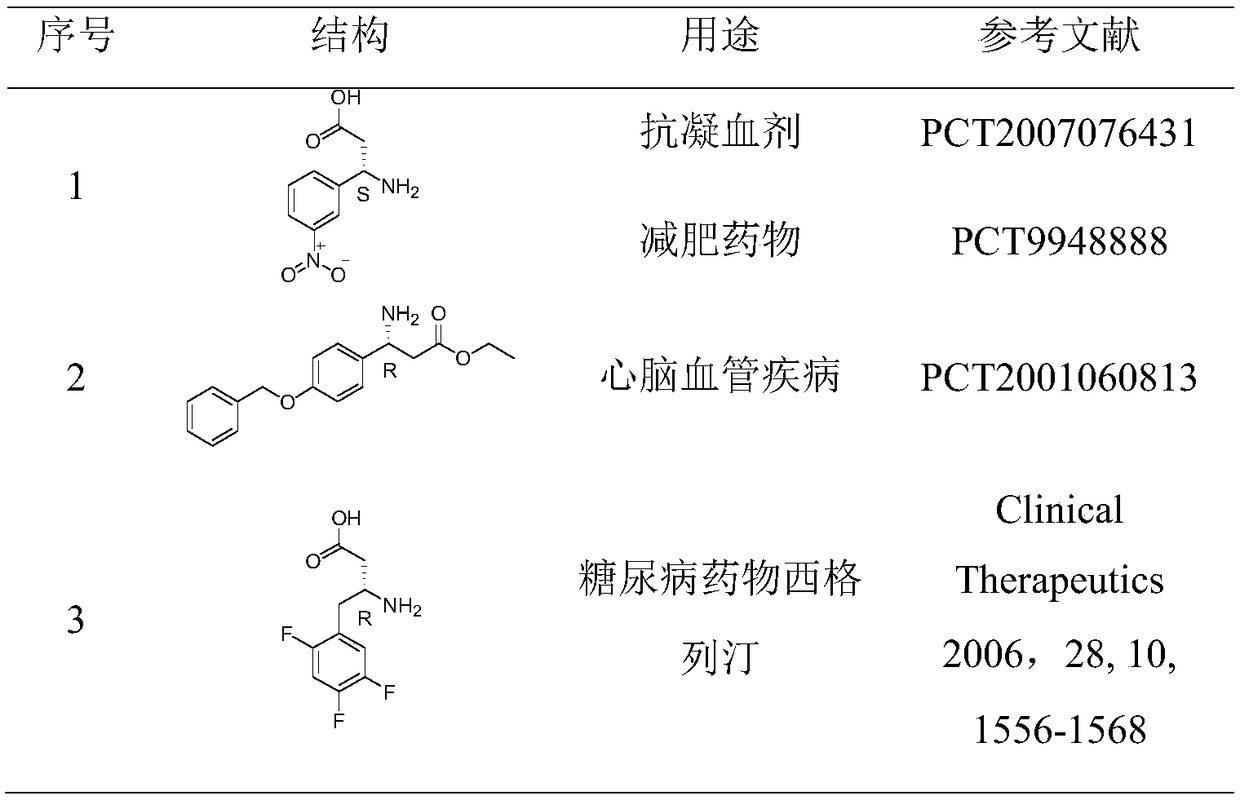
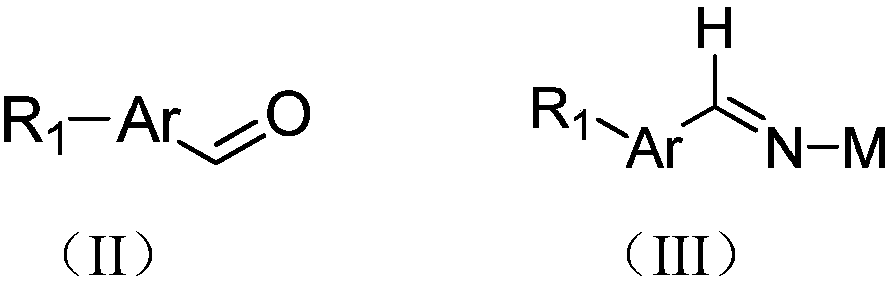Novel efficient method for synthesizing chiral beta-amino acid
An amino acid and chirality technology, applied in organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult removal of heavy metal residues, high cost, limited production capacity, etc., to overcome microbial residues and reaction conditions. Gentle, easy-to-use results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
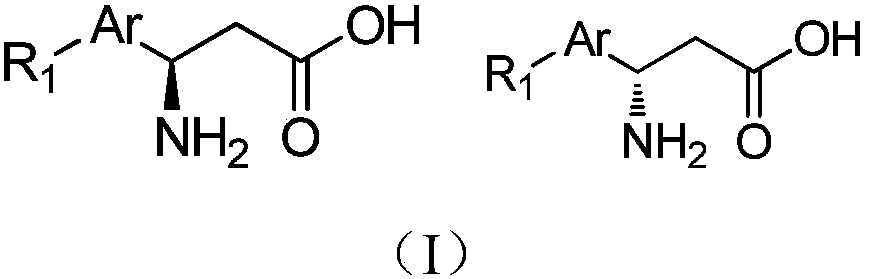
[0030] Synthesis of S-type β-amino acid (I-1) (where R is meta-nitro).
[0031]
[0032] The implementation route is as follows:
[0033]
[0034] Preparation of intermediate (III-1):
[0035]
[0036] 3-Nitrobenzaldehyde (500g, 3.31mol, 1eq), R-tert-butyl sulfinamide (401g, 3.31mol, 1eq), tetraethyl titanate (1.5kg, 6.62mol, 2eq), and then added THF (2.5L), heated to 70°C under reflux overnight while stirring. After TLC showed that the reaction was complete, the reaction solution was poured into 1L saturated brine, extracted twice with ethyl acetate (1L*2), the combined organic phases were dried and concentrated to obtain a yellow thick liquid (610g, crude product), which was directly carried out without further purification. One step reaction.
[0037] Intermediate (IV-1) preparation:
[0038]
[0039] Zinc powder (270g, 4.13mol, 3.5eq) was added into anhydrous THF (1L), under N 2 Heated to 40°C and stirred for 1h in a protected and light-proof environment. ...
Embodiment 2
[0048] Synthesis of R-type β-amino acid (I-2) (where R is p-benzyl ether).
[0049]
[0050] The implementation route is as follows:
[0051]
[0052] Intermediate (III-2) preparation:
[0053]
[0054] 4-Benzyloxybenzaldehyde (200g, 0.94mol, 1eq), S-tert-butyl sulfinamide (113g, 0.94mol, 1eq), tetraethyl titanate (541g, 1.88mol, 2eq) were added to THF (1L), heated to 70°C and refluxed overnight. After TLC showed that the reaction was complete, the reaction solution was added to 1L saturated brine, extracted twice with ethyl acetate (500mL*2), the organic phases were combined, dried and concentrated to obtain a yellow thick liquid (250g, crude product), and proceeded to the next step without purification reaction.
[0055] Preparation of intermediate (IV-2):
[0056]
[0057]Zinc powder (214g, 3.29mol, 3.5eq) was added to anhydrous THF (1L), heated to 40°C and stirred for 1h. After cooling to room temperature, ethyl bromoacetate (314g, 1.88mol, 2eq) was added d...
Embodiment 3
[0066] Synthesis of S-type β-amino acid ester (I-3) (where R is meta-trifluoromethyl).
[0067]
[0068] The implementation route is as follows:
[0069]
[0070] Preparation of intermediate (III-3):
[0071]
[0072] 3-trifluoromethylbenzaldehyde (500g, 2.87mol, 1eq), R-tert-butyl sulfinamide (347g, 2.87mol, 1eq), tetraethyl titanate (1.3kg, 5.74mol, 2eq), Then THF (2.5 L) was added, and heated to 70° C. under reflux overnight while stirring. After TLC showed that the reaction was complete, the reaction solution was poured into 1L saturated brine, extracted twice with ethyl acetate (1L*2), the combined organic phases were dried and concentrated to obtain a yellow thick liquid (713g, crude product), which was directly carried out without further purification. One step reaction.
[0073] Preparation of intermediate (IV-3):
[0074]
[0075] Zinc powder (588g, 9.0mol, 3.5eq) was added to anhydrous THF (2L), heated to 40°C and stirred for 1h. After cooling to room...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com