Sex-determination and methods of specifying same
A sex and male technology, applied in biochemical equipment and methods, animal husbandry, applications, etc., can solve problems such as difficult access to equipment, unsuitable for automation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] Preparation of virus:
[0102] The RCASBP.B virus was modified to carry the open reading frame of GFP, and a recombinant microRNA targeting chicken DMRT1 at the 3'UTR of the open reading frame of GFP. Using the Invitrogen Block-It (Trade Mark) system, Sequence 5 was designed and produced in short hairpin format. The sequence is directed to exon three (exon three) of chicken DMRT1 mRNA, and does not show homology (equal to or greater than 16 / 21 bases) with other sequences in the chicken genome. The hairpin sequence was cloned into an engineered shuttle vector carrying GFP (pRmiR, Figure 5 ). The GFP-microRNA was subsequently subcloned into the RCASBP.B strain. Chicken DF1 cells were then transfected with high quality DNA. Virus was harvested from DF1 cells and purified as previously described (Smith et al, Int. J. Dev. Biol. 53:59-67, 2009). Virus titers of approximately 108 infectious units / mL were obtained. Using the scrambled sequence of the same base as a cont...
Embodiment 1
[0109] Example 1. Down-regulation of DMRT1
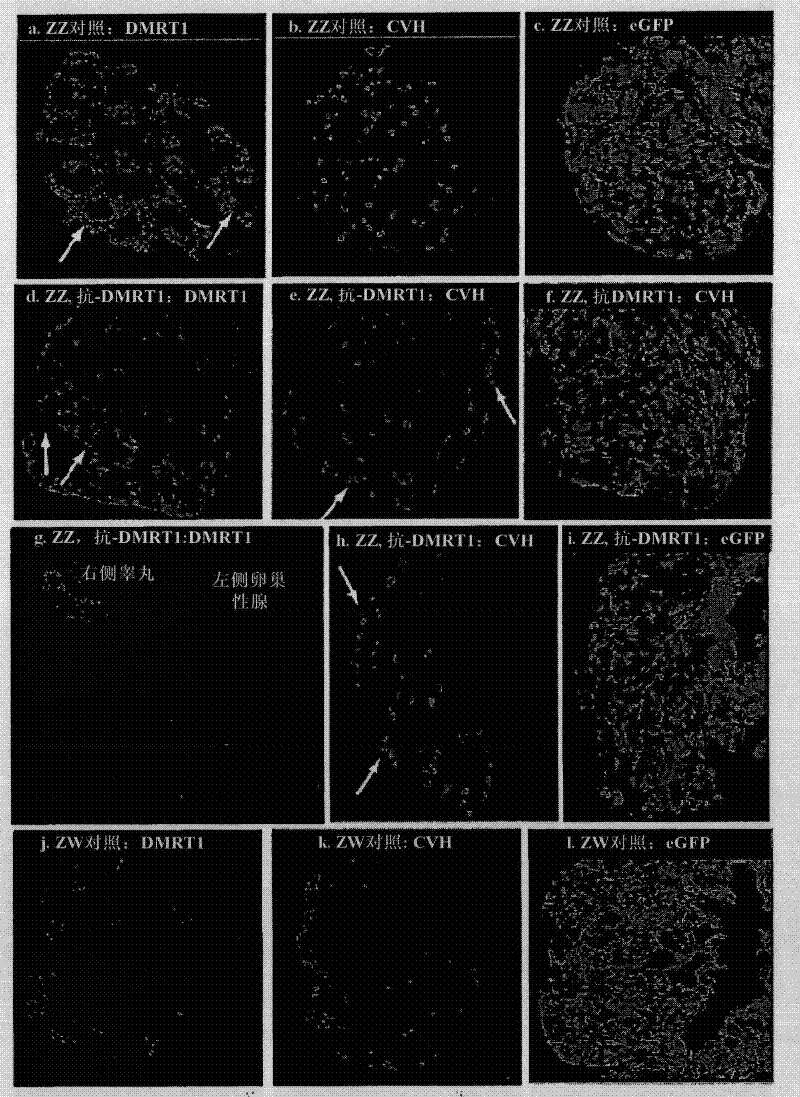
[0110]The recombinant microRNA (miRNA) targeting the DMRT1 transcript was passed through the avian retrovirus vector RCASBP.B (Replication Competent Avian Sarcoma leukosis virus, high titre Bryan Polymerase, strain B, replication competent avian sarcoma leukemia virus, high titer Bryan polymerase , strain B), delivered into live chicken embryos. The virus was engineered to carry the open reading frame of GFP to monitor virus transmission, and the DMRT1 miRNA was located at the 3′UTR of the transgene. Strong viral LTR promoters rather than internal U6 promoters drive miRNA expression. The virus delivered strong GFP expression and knockdown of exogenous DMRT1 protein in cultured chicken DF1 cells ( figure 1 ). Cells infected with the RCASBP.A strain expressing only DMRT1 cDNA showed strong DMRT1 overexpression ( figure 1 a). Cells co-transfected with viruses carrying DMRT1 cDNA and viruses expressing non-silent RCASBP.B.GFP. scra...
Embodiment 2
[0111] Example 2. Generation of Genetically Modified Embryos
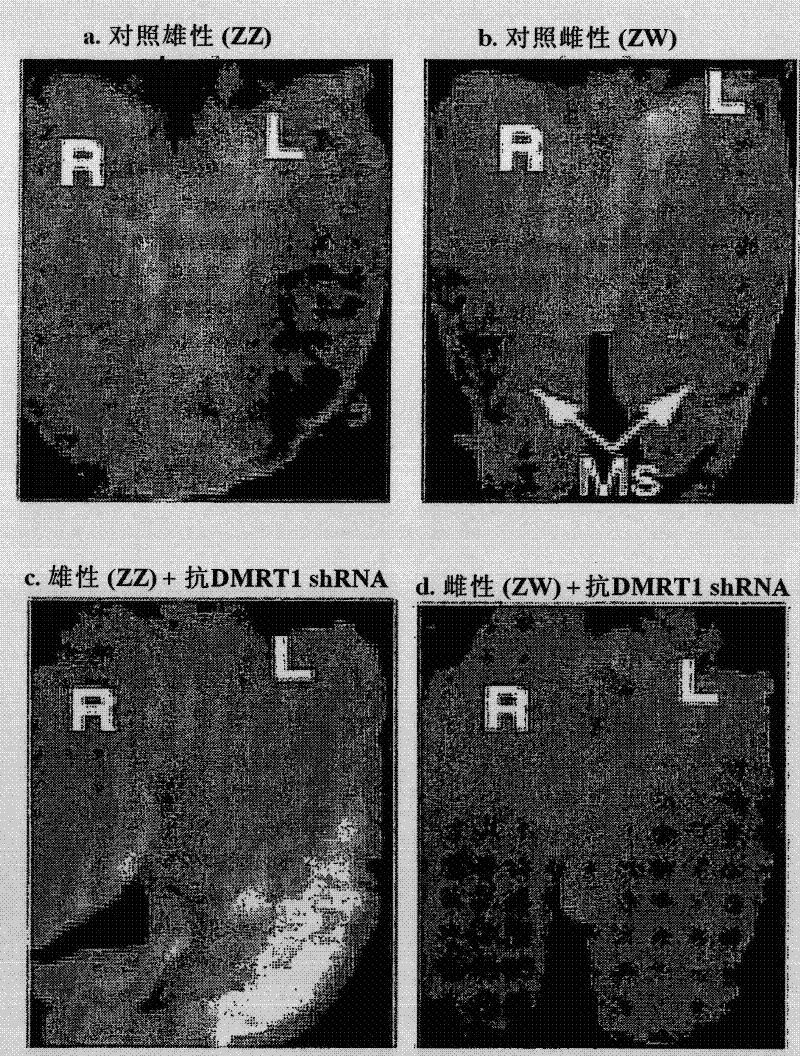
[0112] Day 0 chicken blastoderm was infected with virus carrying a GFP reporter as well as DMRT1 miRNA, and embryogenesis was allowed to proceed until day 10. Control embryos were infected with viruses carrying GFP and scrambled non-silent miRNA sequences. All embryos were genotypically sexed by PCR amplification of W (female) specific Xho1 repeats. In chicken embryos, gonads form on the mesonephros around day 3.5 of incubation. On day 6, sexual differentiation to the testis or ovary begins and usually proceeds to day 10. Embryos infected with virus at day 0, by day 10, showed strong global expression of GFP, including widespread expression in the urinary system and in the 25 gonads sectioned. General embryonic development is normal. Gonadal development of embryos treated with the control miRNA was normal, with bilaterally symmetrical testes in males and typical asymmetrical ovarian development in females ( f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com