Valsartan solid dispersion and preparation method thereof
A technology of solid dispersion and valsartan, which is applied in the field of medicine, can solve the problems of low drug solubility, achieve the effect of improving the dissolution rate and solving the problem of organic solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
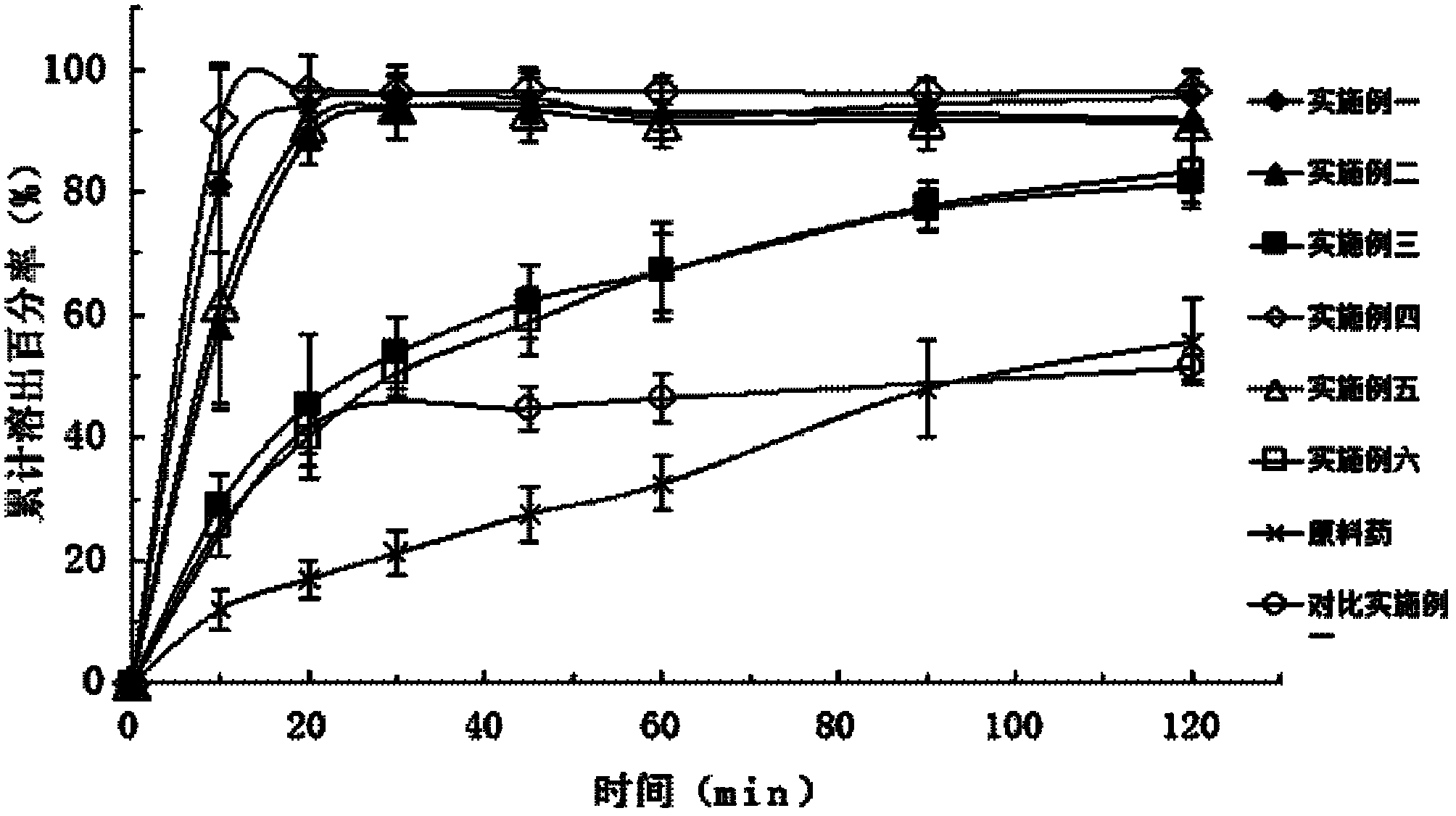
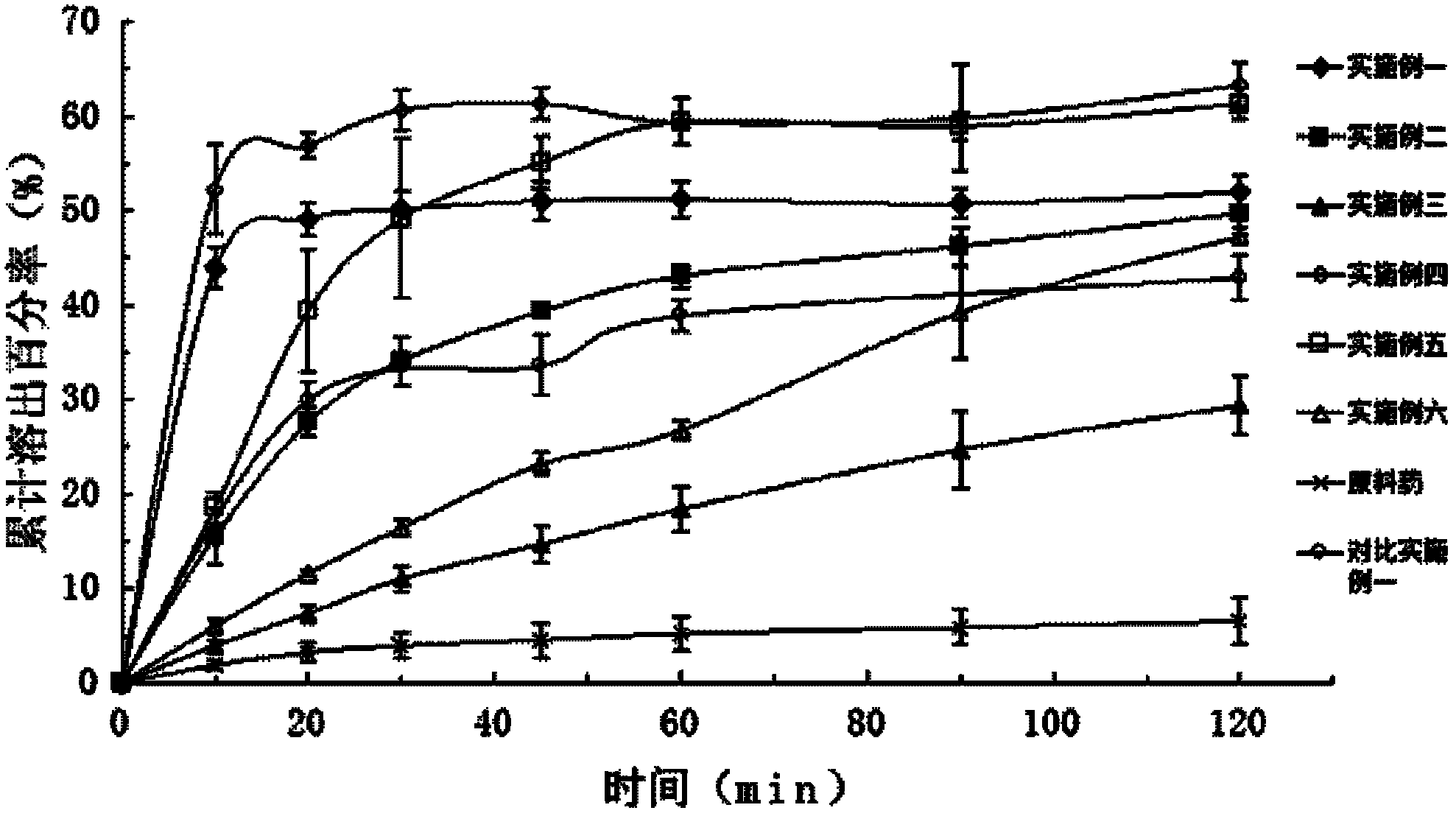
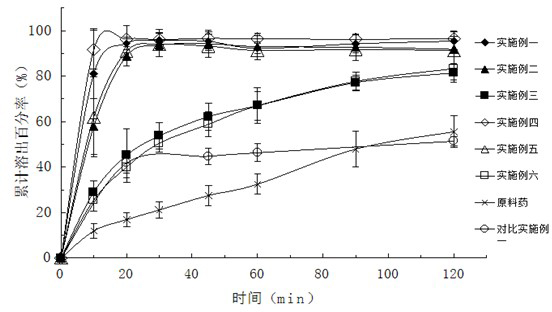
[0037] Embodiment one: the preparation of valsartan solid dispersion
[0038] Put 800mg of valsartan and 2400mg of polyethylene glycol 6000 melted in a water bath at 50-60°C in a beaker, add 5ml of 0.8mol / L sodium hydroxide solution and stir, and add an appropriate amount of distilled water and stir until the valsartan and high The molecules are all dissolved in water to form a clear solution, which is freeze-dried until the sample is completely dried to form a solid dispersion.
Embodiment 2
[0039] Embodiment two: the preparation of valsartan solid dispersion
[0040]Put 800mg valsartan and 2400mg polyvinylpyrrolidone K-30 in a beaker, add 5ml of 0.8mol / L sodium hydroxide solution to stir, and add appropriate amount of distilled water to stir until both valsartan and polymer are dissolved in water to form Clarify the solution and freeze-dry until the sample is completely dry to form a solid dispersion.
Embodiment 3
[0041] Embodiment three: the preparation of valsartan solid dispersion
[0042] Put 800mg valsartan and 2400mg hypromellose in a beaker, add 5ml of 0.8mol / L sodium hydroxide solution to stir, and add appropriate amount of distilled water to stir until valsartan and polymer are dissolved in water to form a clear The solution was freeze-dried until the sample was completely dried to form a solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com