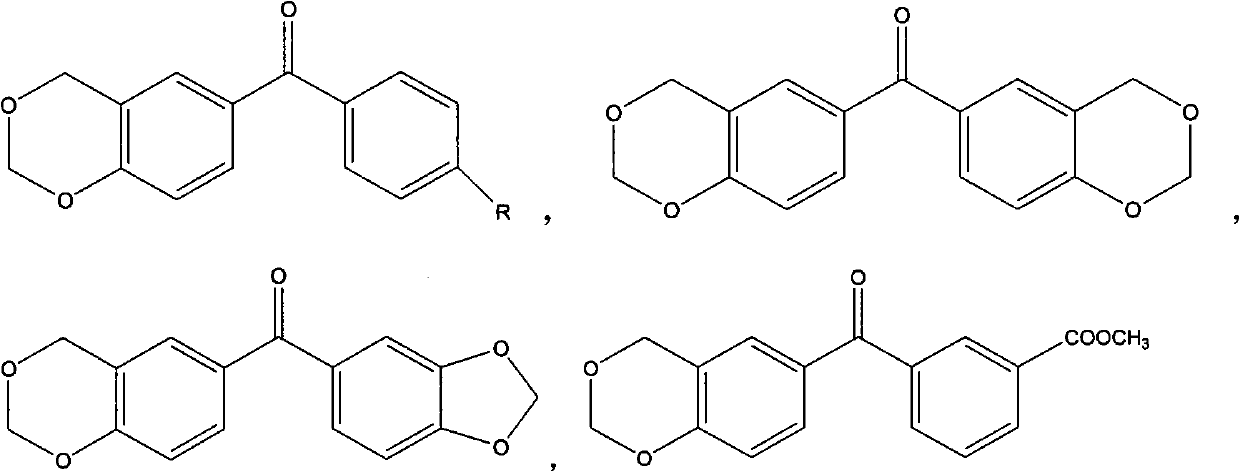
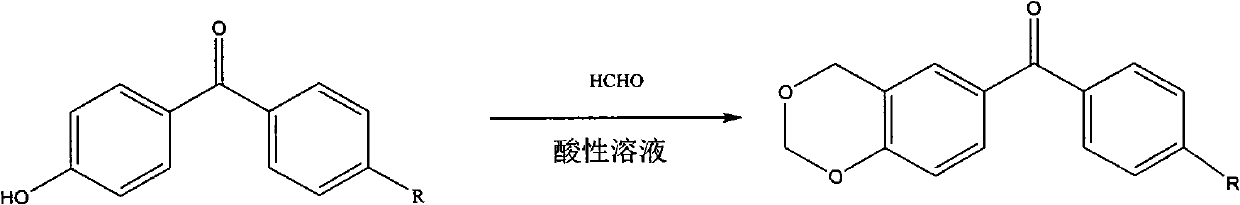
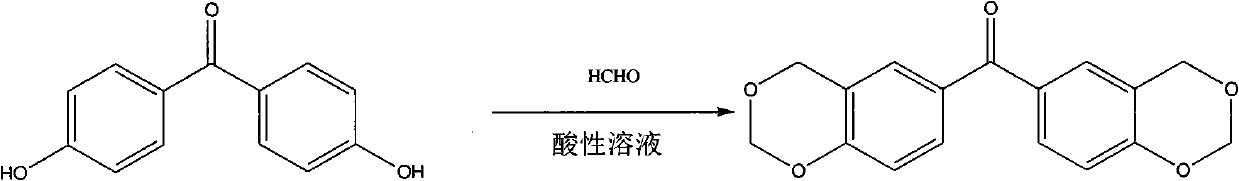
Benzophenone photoinitiator containing cyclic acetal and method for preparing same
A technology of benzophenone and photoinitiator, which is applied in the field of photoinitiator and its preparation, can solve problems such as restricted application, toxicity and carcinogenicity, and easy yellowing of cured film, so as to reduce yellowing and toxicity, and reduce production cost Low, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 0.02mol (3.96g) of 4-hydroxybenzophenone to 28mL of 37% formaldehyde solution at room temperature, slowly add 150mL of hydrochloric acid and 30mL of phosphoric acid mixed acid solution dropwise for more than 2 hours, and slowly heat up after the dropwise addition , stirred at 48°C for 6 hours, cooled to room temperature, filtered, washed the filter cake with water until neutral, dried, recrystallized with a mixed solvent of ethanol and water (10:3v / v), dried in vacuo to obtain 1,3- Dioxybenzohexane benzophenone 4.15g, yield 86.5%.
Embodiment 2
[0022] Add 0.02mol (3.96g) of 4-hydroxybenzophenone to 28mL of 37% formaldehyde solution at room temperature, slowly add 150mL of hydrochloric acid, 10mL of mixed acid solution of perchloric acid for more than 2 hours, after the dropwise addition Slowly heat up, stir at 50°C for 8 hours, cool to room temperature, filter, wash the filter cake with water until neutral, dry, recrystallize with a mixed solvent of ethanol and water (10:2v / v), and dry in vacuo to obtain 1, 3.24 g of 3-dioxobenzohexane benzophenone, yield 67.5%.
Embodiment 3
[0024] Add 0.02mol (4.24g) of 4-hydroxy-4'methylbenzophenone to 28mL of 37% formaldehyde solution at room temperature, slowly add 100mL of hydrochloric acid and 20g of p-toluenesulfonic acid mixed acid solution for more than 2 hours After the dropwise addition, the temperature was raised slowly, stirred at 10°C for 6 hours, filtered, the filter cake was washed with water until neutral, dried, recrystallized with a mixed solvent of ethanol and water (10:1 v / v), dried in vacuo to obtain 1 , 3.68 g of 3-dioxobenzohexane-4'methylbenzophenone, yield 72.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com