Propofol hydroxy acid ester compound with ester constitutional terminal, preparation method for same and application thereof
A technology of propofol hydroxy acid and ester compounds, which is applied in the field of propofol derivatives, and can solve the problems of inability to exert pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Acetate of Propofol Hydroxybutyrate:
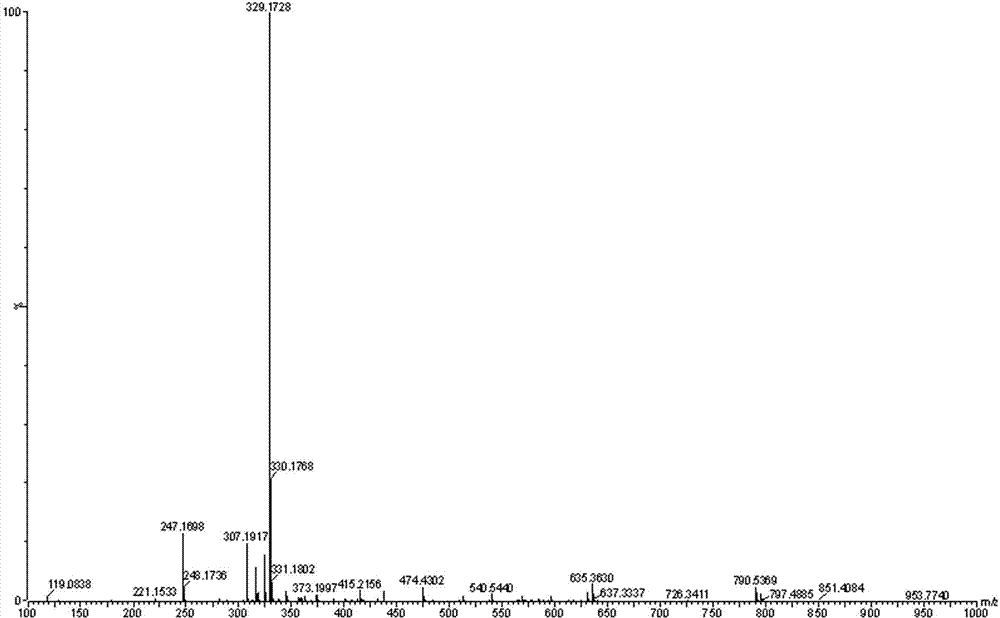
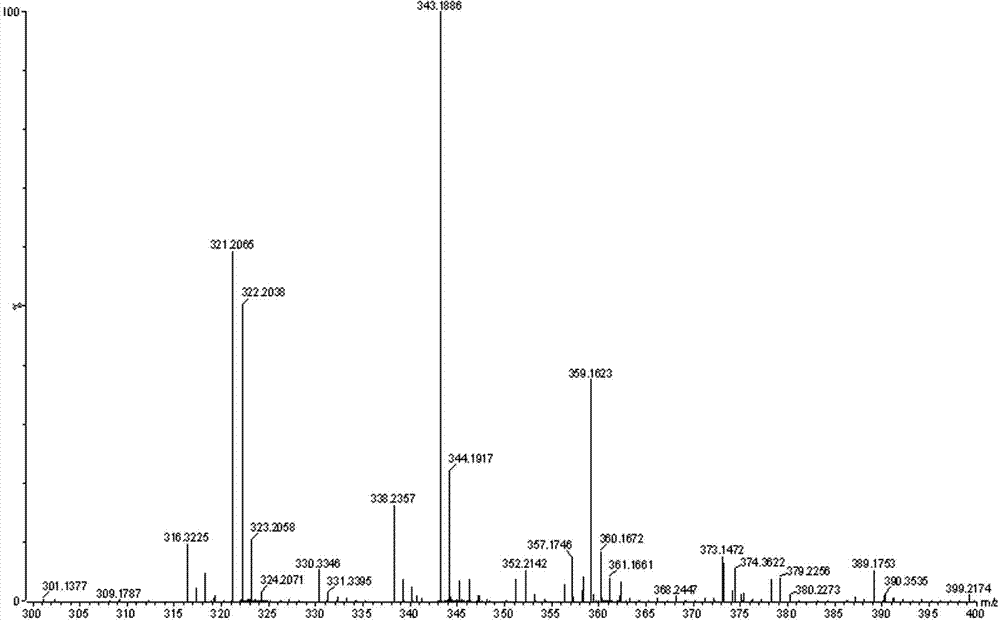
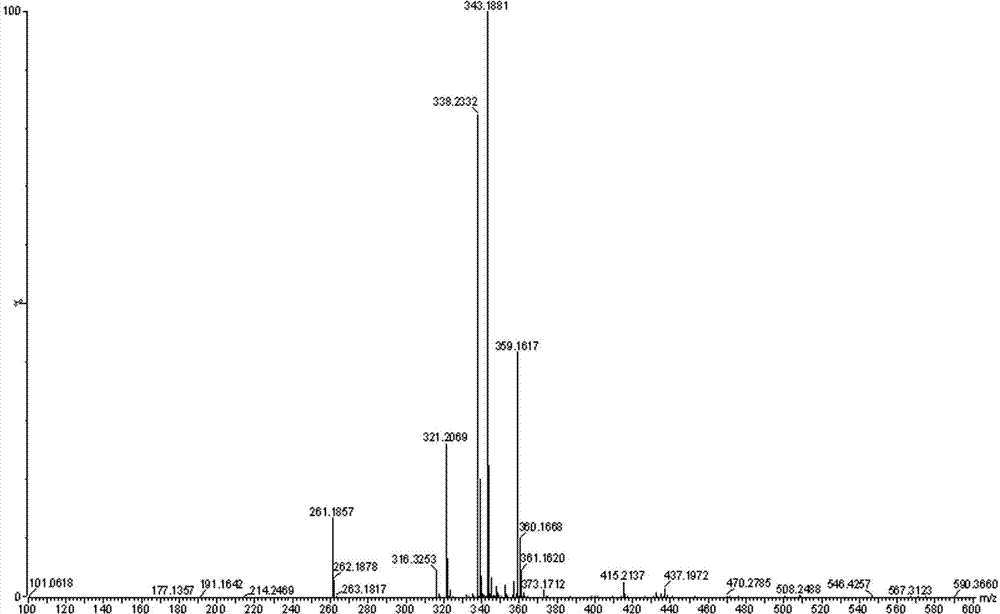
[0025] Dissolve 4 g of propofol 4-hydroxybutyrate prepared in the manner of publication number CN101906039A in 20 ml of dichloromethane, add 10 ml of acetic anhydride, cool to 0°C, add dropwise 3 ml of triethylamine, and keep the temperature Stir the reaction for 24 h. After the reaction is complete, pour the reaction solution into 100 ml of water, stir for 10 min, let stand to separate layers, separate the organic layer, wash the organic layer once with saturated sodium carbonate solution and water, separate the organic layer, and reduce After concentration under pressure, it was separated and eluted by column chromatography (eluent (v / v, the same below): cyclohexane / ethyl acetate=95 / 5), and the eluent containing the product was evaporated to remove the solvent. 3.7 g of a colorless oil was obtained, with a yield of 79.7%. Product structure detection results:
[0026] 1) Nuclear Magnetic Resonance Apparatus: BRUKE...
Embodiment 2
[0034] Preparation of Acetate of Propofol Hydroxybutyrate:
[0035] Dissolve 5 g of propofol 4-hydroxybutyrate in 15 ml of acetic anhydride, add 3 ml of triethylamine dropwise, and stir at room temperature for 3 h. After the reaction is completed, pour the reaction solution into 100 ml of water, add 50 ml of ethyl acetate ester, stirred for 10 min, allowed to stand and separate the layers, separated the organic layer, washed the organic layer once with saturated sodium carbonate solution and water, separated the organic layer, concentrated under reduced pressure and eluted by column chromatography, the eluted product containing After distilling off the solvent under reduced pressure, 4.84 g of a colorless oil was obtained, with a yield of 83.5%.
Embodiment 3
[0037] Preparation of Acetate of Propofol Hydroxybutyrate:
[0038] Dissolve 5 g propofol 4-hydroxybutyrate in 15 ml acetic anhydride, add 3 ml triethylamine dropwise, stir and react at 50°C for 1 h, after the reaction is completed, pour the reaction solution into 100 ml water, add 50 ml Ethyl acetate, stirred for 10 min, allowed to stand for stratification, separated the organic layer, washed the organic layer once with saturated sodium carbonate solution and water, separated the organic layer, concentrated under reduced pressure and eluted by column chromatography, the product containing After the solvent was evaporated from the eluent under reduced pressure, 4.25 g of a colorless oily substance was obtained, with a yield of 73.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com