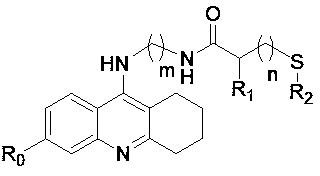
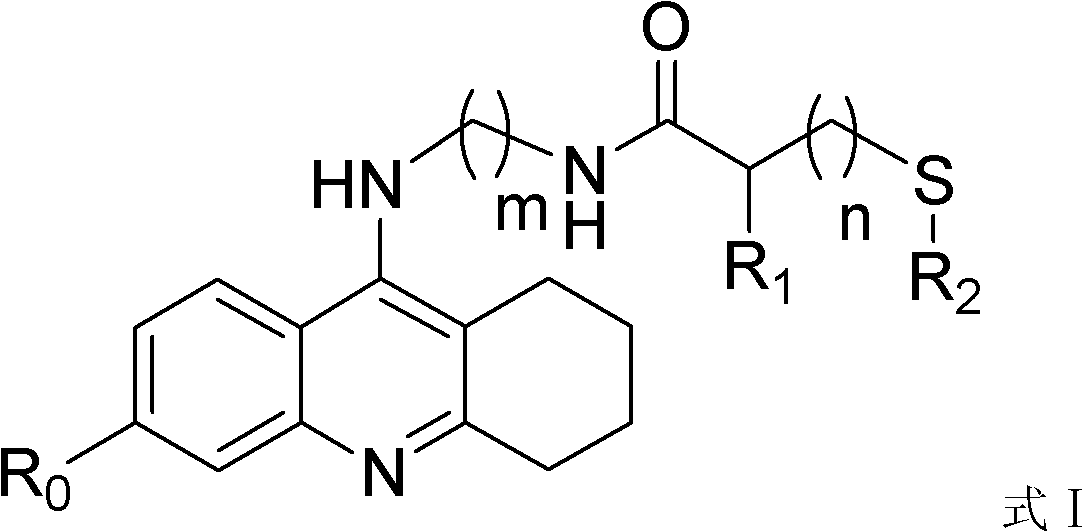
Sulfydryl-containing tacrine derivative and preparation method and application thereof
A technology of tacrine and derivatives, which is used in medical preparations, pharmaceutical formulations, and drug combinations containing active ingredients to achieve the effects of improving learning and memory, improving learning and memory ability, and overcoming liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of 3-(thioacetyl)propionic acid
[0050] 20.8ml (0.29mol) of thioacetic acid was dissolved in 20ml of tetrahydrofuran, slowly added dropwise to the tetrahydrofuran solution containing 16ml (0.224mol) of 3-bromopropionic acid, and 29.0g of triethylamine was added as a catalyst. The temperature was controlled at 50°C and the reaction was stirred for 20 hours. Most of the tetrahydrofuran was removed by rotary evaporation, and the obtained colorless viscous liquid was left to stand for crystallization for 24 hours to obtain colorless needle-like crystals. Suction filter and wash the filter cake with a small amount of tetrahydrofuran, and dry under reduced pressure to obtain 13.0 g of colorless massive crystals with a yield of 39.2%. 1 H-NMR (DCCl 3 , 400MHz, δ / ppm): 11.0(s, 1H), 3.08(t, J=7.2Hz, 2H), 2.66(t, J=7.2Hz, 3H), 2.32(s, 3H).
Embodiment 1-1
[0051] Embodiment 1-1: 2-(thioacetyl) propionic acid can be prepared in a method similar to that of Example 1:
[0052] 2.28g (0.03mol) of thioacetic acid was dissolved in 10ml of tetrahydrofuran, slowly added dropwise to a solution containing 4.56g (0.03mol) of 2-bromopropionic acid in tetrahydrofuran, and 3.0g of triethylamine was added as a catalyst. The temperature was controlled at 50°C and the reaction was stirred for 20 hours. The precipitated crystals in the reaction solution were filtered off with suction, and most of the tetrahydrofuran was removed by rotary evaporation of the filtrate to obtain 2.4 g of a yellow liquid with a yield of 54%. 1 H-NMR (DCCl 3 , 400MHz, δ / ppm): 12.99(s, 1H), 4.02(q, J=7.2Hz, 1H), 2.32(s, 3H), 1.37(d, J=7.2Hz, 3H).ESI-MS: [M-1] - =146.8.
Embodiment 2
[0053] Example 2 Preparation of 3-thioacetyl-N-((5,6,7,8-tetrahydroacridinyl-9-amino)propylamino)propionamide
[0054]
[0055] Weigh 0.444g (0.003mol) of 3-(thioacetyl)propionic acid and dissolve it in 10ml of dichloromethane, add 0.7g of HOBt, stir at 20°C for 10min, then add 1.0g of EDC·HCl, and then The temperature was controlled at 25°C and stirred for 30 minutes, and then 0.84 g (0.0033 mol) of 9-(3-aminopropylamino)-5,6,7,8-tetrahydroacridine was added. Control the temperature at 25°C and stir for 18 hours to stop the reaction, add saturated brine to the reaction system, then wash the reaction solution with 0.5M dilute acid, separate the liquid and then wash it with saturated NaHCO 3 The dichloromethane layer was washed with the solution, the layers were separated and the dichloromethane layer was washed again with saturated brine. Separation of the obtained dichloromethane layer with anhydrous magnesium sulfate, direct column chromatography purification (dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com