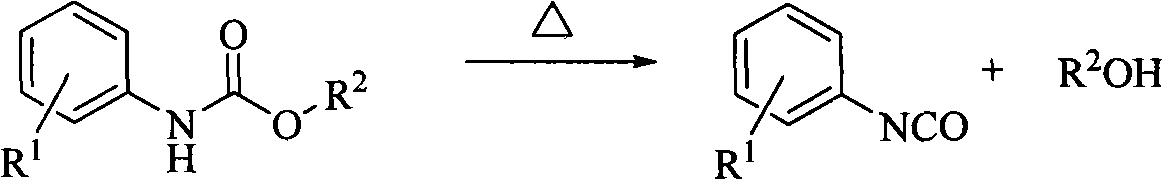
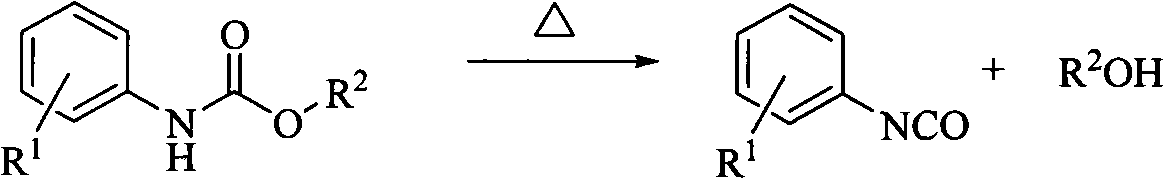
Method for preparing (substituted) aromatic monoisocyanate from (substituted) aryl carbamate through thermal decomposition
A technology of aryl monoisocyanate and aryl carbamate, applied in carbamate preparation, organic chemistry, etc., can solve the problems of by-products, achieve mild reaction conditions, high conversion rate of raw materials, and high energy utilization rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 20.0 g of methyl phenylcarbamate was added into a 50 ml flask, and 0.1 g of catalyst ZnO was added at the same time, and the amount added was 0.5% of the mass of methyl phenylcarbamate. Under the condition of vacuum degree of 450mmHg, the reaction temperature was raised to 200°C, the condensed water temperature was 20°C, and the reaction was carried out for 120min. After the reaction, the fraction was treated with di-n-butylamine and analyzed by HPLC. The phenylisocyanate in the fraction was 13.26g , the yield was 84.1%.
Embodiment 2
[0022] In a 50ml flask, add 20.0g of methyl phenylcarbamate, while adding 0.2g of catalyst Co 2 o 3 , the addition amount is 1% of the quality of methyl phenylcarbamate. Under the condition of vacuum degree of 480mmHg, the reaction temperature was raised to 200°C, the condensed water temperature was 20°C, and the reaction was carried out for 100min. After the reaction, the fraction was treated with di-n-butylamine and analyzed by HPLC. The phenylisocyanate in the fraction was 13.81g , the yield was 87.6%.
Embodiment 3
[0024] Add 25.0 g of ethyl phenylcarbamate to a 50 ml flask, and at the same time add 0.2 g of catalyst neutral Al 2 o 3 , the addition amount is 0.8% of the mass of ethyl phenylcarbamate. Under the condition of vacuum degree of 540mmHg, the reaction temperature was raised to 220°C, the condensed water temperature was 40°C, and the reaction was carried out for 120min. At the end of the reaction, the fraction was treated with di-n-butylamine and analyzed by HPLC. The phenylisocyanate in the fraction was 14.09g , and the yield was 76.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com