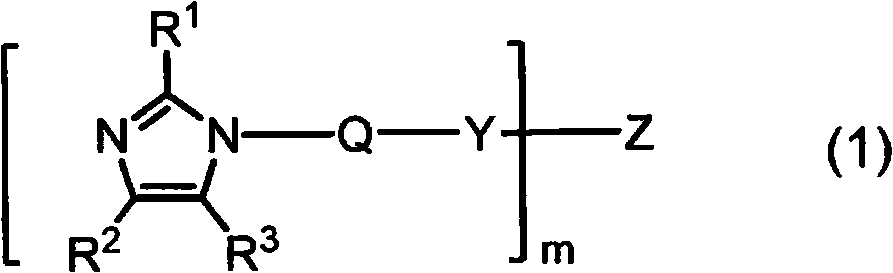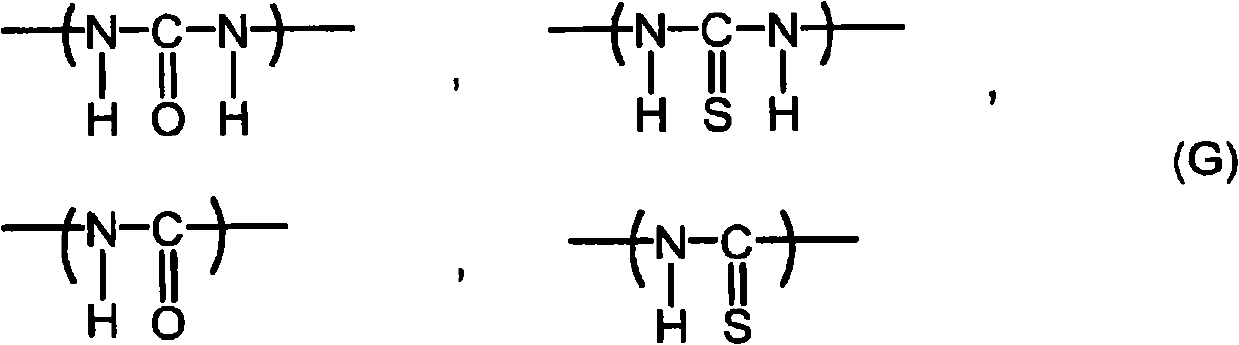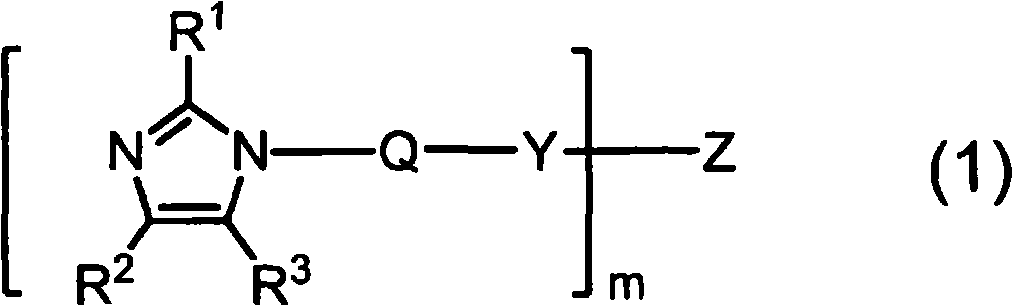Imidazole compound-containing microcapsulated composition, curable composition using same, and masterbatch type curing agent
A curable composition and compound technology, which is applied in the field of masterbatch-type curing agent and curable composition, can solve the problems of increased compounding frequency, reduced operating efficiency, and limited effective use of the composition, and achieves excellent low-temperature curability , high solvent stability, and high storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0234] Dissolve 30.0 g (239.67 mmol, manufactured by Shikoku Chemicals Co., Ltd.) of 1-aminoethyl-2-methylimidazole in 600 mL of acetonitrile, and add dropwise 17 mL of ( 105.92 mmol, Wako Pure Chemical Industries, Ltd.) 1,6-hexamethylene diisocyanate. During the dropwise addition, a white solid started to form and became a slurry. After the dropwise addition, the mixture was stirred at room temperature for 3 hours, and the resulting white solid was collected by filtration and dried under reduced pressure to obtain 41.67 g (yield 94%) of an imidazole compound represented by the following formula (6). The obtained imidazole compound was identified by proton nuclear magnetic resonance spectrum and infrared absorption spectrum. In addition, the low-molecular-weight amine compound present together with the imidazole compound represented by the formula (6) was quantified by the method of the above-mentioned (3) using HPLC.
[0235]
[0236] The obtained white solid was pulveri...
Embodiment 2
[0240] Dissolve 30.0 g (239.67 mmol, manufactured by Shikoku Chemicals Co., Ltd.) of 1-aminoethyl-2-methylimidazole in 600 mL of acetonitrile, and add dropwise 81 mL of ( 235.74 mmol, manufactured by Wako Pure Chemical Industries, Ltd.) n-octadecyl isocyanate. After the dropwise addition, the mixture was stirred at room temperature for 3 hours, and the resulting white solid was collected by filtration and dried under reduced pressure to obtain 93.21 g (yield 94%) of an imidazole compound represented by the following formula (7). The obtained imidazole compound was identified by proton nuclear magnetic resonance spectrum and infrared absorption spectrum. In addition, the low-molecular-weight amine compound present together with the imidazole compound represented by the formula (7) was quantified by the method of the above-mentioned (3) using HPLC.
[0241]
[0242] The obtained white solid was pulverized in the same manner as in Example 1 to obtain a finely powdered imidazo...
Embodiment 3
[0244] Dissolve 30.0 g (239.67 mmol, manufactured by Shikoku Chemicals Co., Ltd.) of 1-aminoethyl-2-methylimidazole in 600 mL of acetonitrile, and add dropwise 30 mL of ( 237.28 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.) cyclohexyl isocyanate. After the dropwise addition, the mixture was stirred at room temperature for 3 hours, and the resulting white solid was collected by filtration and dried under reduced pressure to obtain 55.24 g (yield 93%) of an imidazole compound represented by the following formula (8). The obtained imidazole compound was identified by proton nuclear magnetic resonance spectrum and infrared absorption spectrum. In addition, the low-molecular-weight amine compound present together with the imidazole compound represented by the formula (8) was quantified by the method of the above-mentioned (3) using HPLC.
[0245]
[0246] The obtained white solid was pulverized in the same manner as in Example 1 to obtain a finely powdered imidazole ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com