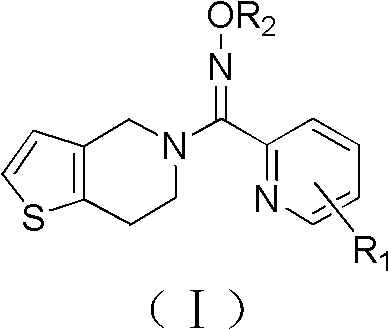
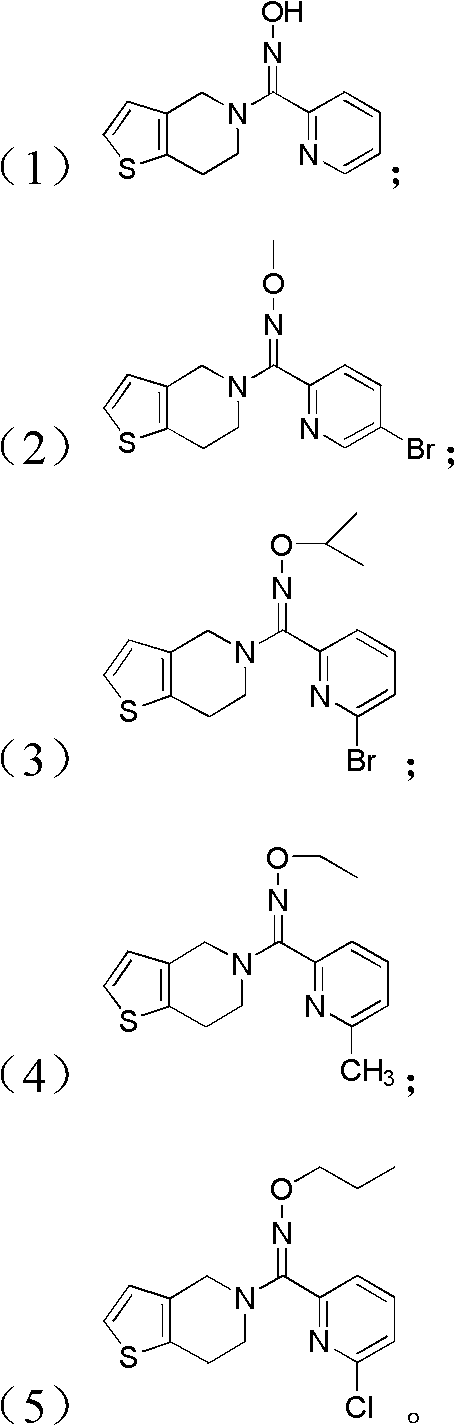
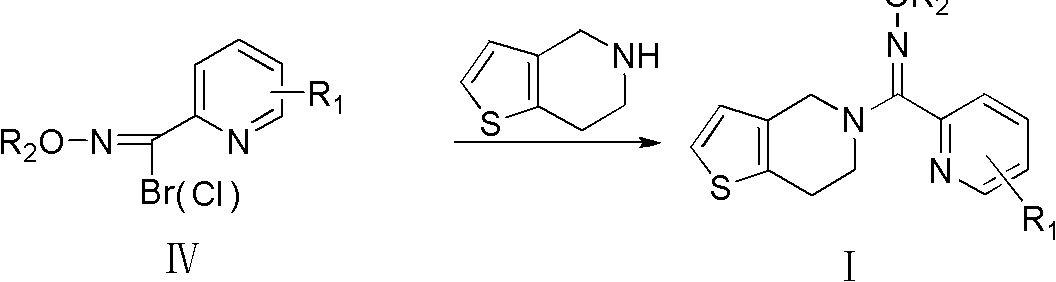
Pyridine derivatives, preparation method thereof, and purpose thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as injury, side effects, and low cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0059] Synthesis of Intermediate IV-1
[0060]
[0061] Add 12.2 g of intermediate III-1 to a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 40 mL of dichloromethane, and add 17.8 g of NBS while stirring. React under light at room temperature for 6 hours (the plate layer shows that the reaction is complete). with 3 x 30mL 35% Na 2 S 2 o 3 The reaction solution was washed with aqueous solution, and the dichloromethane layer was fully dried with anhydrous sodium sulfate, filtered, and the dichloromethane was evaporated under reduced pressure to obtain a light yellow oily product (HPLC: 96.2%). Rf = 0.36 [single site, developing solvent: v (dichloromethane): v (methanol) = 5: 1].
[0062] Referring to the method of Reference Example 2, intermediates IV-2 to IV-5 can be synthesized.
[0063] Table 3 List of Intermediates IV-2~IV-5
[0064]
Embodiment 1
[0066] Synthesis of (6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)(pyridin-2-yl)methanone oxime (Compound I-1)
[0067]
[0068] Add 10.0 g of intermediate IV-1 into a reaction flask equipped with stirring, a condenser, and a thermometer, dissolve it with 30 mL of anhydrous methanol, and add 6.5 g of anhydrous potassium carbonate while stirring. 7.0 g of 4,5,6,7-tetrahydrothieno[3,2-c]pyridine was added to the reaction system in batches. After the addition was completed, the reaction was continued for 3.5h under reflux (the plate showed that the reaction was complete). Filter out the solid matter, evaporate anhydrous methanol to dryness, wash the reaction solution with 3×30mL water, extract with dichloromethane, fully dry with anhydrous sodium sulfate, filter, and evaporate dichloromethane under reduced pressure to obtain a black oil. Column separation [mobile phase: v (dichloromethane): v (methanol) = 5: 1], Rf = 0.45, gave a light yellow solid (HPLC: 99.3%).
[0069] Referr...
Embodiment 3
[0075] Formation of compound I-2 into taurate: Take 2.0 g of the white solid product of compound I-2 and dissolve it in 10 mL of anhydrous methanol. After heating to reflux, equimolar taurine was added, and the reaction was stirred under reflux for about 1.5h. After the reaction was completed, it was left to stand at room temperature for 24 h. Transparent crystals were precipitated, filtered and dried in vacuo.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com