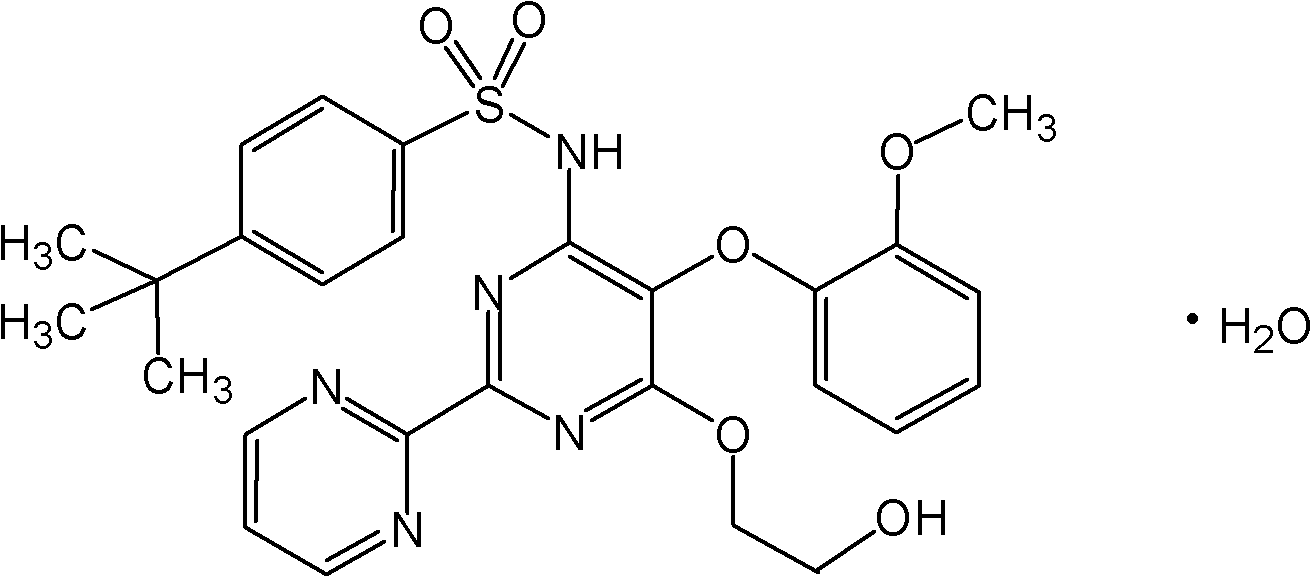
Process for preparation of endothelial receptor antagonist (bosentan)
A technology of bosentan and solvent, applied in the field of preparing endothelin receptor antagonists, can solve the problems of expensive and cumbersome methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0040] According to a preferred embodiment of the present invention, the method for preparing bosentan comprises reacting (2-methoxyphenoxy)-2,2'-bipyrimidine (compound 1) with phosphorus oxychloride to obtain 4,6-bis Chloro-5-(2-methoxyphenoxy)-2,2'-bipyrimidine (Compound 2). This intermediate is refluxed with 4-tert-butylbenzenesulfonamide (compound 3) in the presence of a base such as alkali metal hydroxide or carbonate and solvent to give 4-tert-butyl-N-[6-chloro- 5-(2-Methoxyphenoxy)-4-pyrimidinyl]benzenesulfonamide (Compound 4). The product is reacted with ethylene glycol in the presence of alkali metal amide or alkali metal hydride to obtain crude bosentan. The obtained product was purified with an organic solvent such as methanol and isopropyl acetate. This embodiment is shown in Scheme I below.
[0041]
[0042]
[0043] Process I
[0044] In embodiments of the present invention, solvents used include, but are not limited to, acetone and toluene.
[0045] B...
Embodiment 1
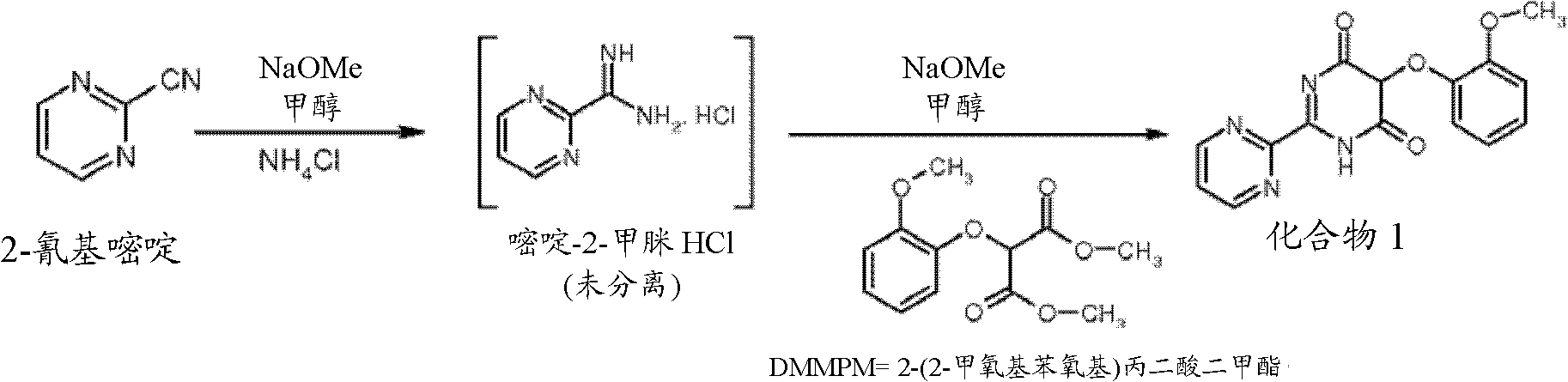
[0086] 5-(2-Methoxyphenyl)-2-(pyrimidin-2-yl)pyrimidine-4,6-(1H,5H)-dione from 2-cyanopyrimidine (compound 1)
[0087]900ml of methanol and 100.0g of 2-cyanopyrimidine were added at 25-30°C and stirred for 5 minutes. A solution of 5.14 g of sodium methoxide in 50.0 ml of methanol was added at 25-30° C. and stirred for 3.0 hours. The progress of the reaction was monitored by HPLC. Add 56.0 g of ammonium chloride and stir at 25-30°C for 3.0 hours. A stock solution of 221.0 g of sodium methoxide in 800.0 ml of methanol (weight - 781.0 gm) was prepared. 599.0 g of sodium methoxide was added to the reaction mass at 20-25°C. Stir at 20-25°C for 1.0 hour. The reaction was cooled to 20 °C. A stock solution of 338.50 g of DMMPM in 1.60 L of methanol was prepared (weight - 1.5475 kg). 1.216 kg of the prepared stock solution of DMMPM was added to the reactant at 20-25°C. Stir at 20-25°C for 7.0 hours. The remaining sodium methoxide stock solution in methanol (182.0 gm) from t...
Embodiment 2
[0089] From 5-(2-methoxyphenyl)-2-(pyrimidin-2-yl)pyrimidine-4,6-(1H,5H)-dione (compound 1) 4,6-Dichloro-5-(2-methoxyphenoxy)-2,2'-bipyrimidine (compound 2)
[0090] 343.70 g of phosphorus oxychloride was added, followed by 175.0 g of compound 1. The temperature of the reaction was raised to reflux. The reaction was stirred at reflux for 4.0 hours. The reaction was monitored by HPLC. The reaction was gradually cooled to 40-50°C. The reaction was quenched with 2.625 L of water at 5-10°C. The reaction was stirred at 5-10°C for 2.0 hours. Filter and wash the wet material three times with 175.0 ml of water. Unload wet material. Weight of wet compound 2: 255 g. The wet mass was vacuum dried at 55-60°C for 8.0 hours. Weight of dry compound 2: 182g (% yield: 93%; HPLC purity: >98%))
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com