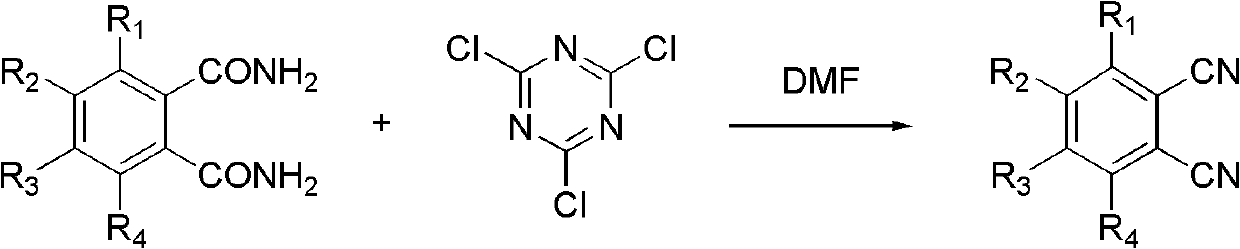
Synthetic method of benzene dinitrile or substituted benzene dinitrile
A synthesis method, the technology of phthalonitrile, which is applied in the field of synthesis of phthalonitrile or substituted phthalonitrile, can solve the problems of large environmental impact and unsuitability for large-scale production, and achieve low environmental impact, low production pollution, and cost reduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
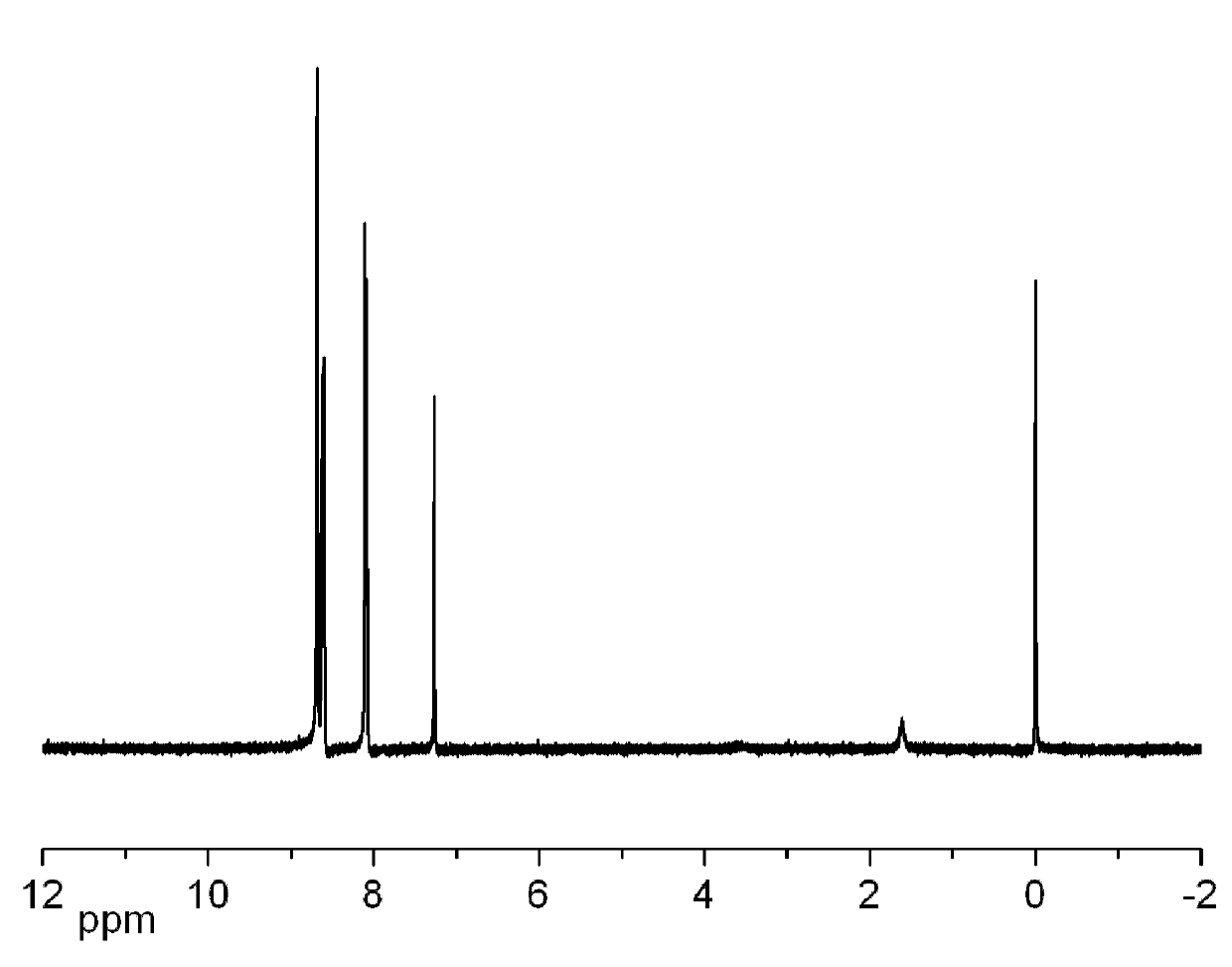
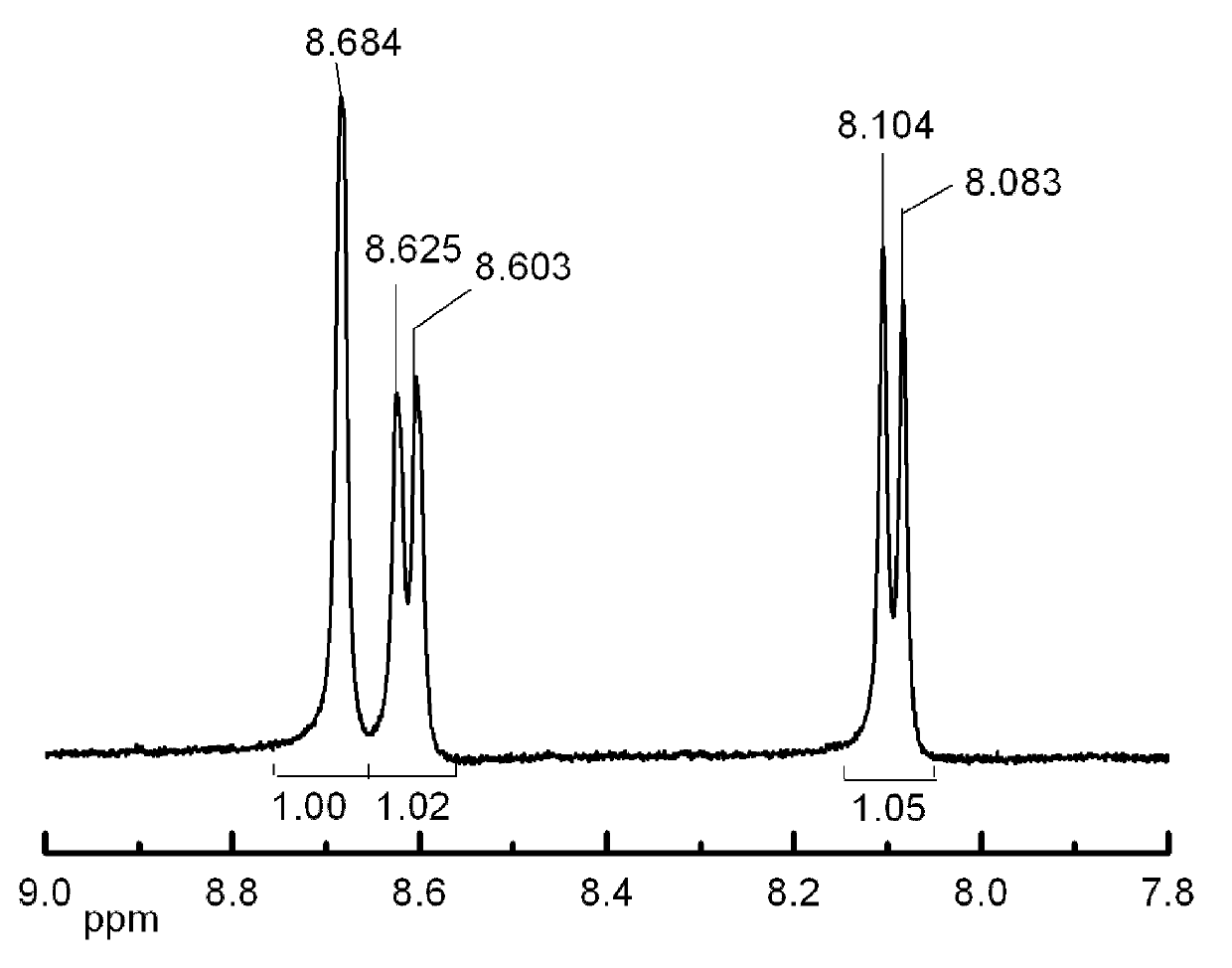
[0029] 37g of 4-nitrophthalamide and 350mL of DMF were successively added to a 500mL single-necked round-bottomed flask, placed on a magnetic stirrer and stirred for 30min, the system was placed in an ice bath, and when the temperature of the system dropped below 5°C, added 42.5 g of cyanuric chloride was connected to the liquid sealing device, and after stirring for 20 min, the ice bath was removed, and the stirring was continued at room temperature for 48 hours. The system was placed in an ice bath, cooled to 0 °C, 50 mL of distilled water was added dropwise with a constant pressure funnel, and the rate of addition was controlled to keep the temperature of the system below 15 °C. After the system was clarified, pour it into 1.0 L of ice water. in the mixture. After filtration, washing with water, and drying, 29 g of 4-nitrophthalonitrile were obtained, the yield was about 95%, and the melting point was 141-144°C. The hydrogen NMR data of the target product are as follows f...
Embodiment 2
[0031] 10g 4-nitrophthalamide and 100mL DMF were successively added to a 250mL single-necked round-bottomed flask, the system was stirred on a magnetic stirrer, the temperature of the system was lowered to 15°C, 11.5g of cyanuric chloride was added, and the mixture was continuously stirred. When the liquid sealing device was installed, the system exothermed violently, and a large number of bubbles were generated, and the reaction was completed by continuing to stir at this temperature for 5 hours. It was then poured into 500 mL of ice-water mixture. Filter, wash with water, and dry to obtain 8 g of 4-nitrophthalonitrile with a yield of about 97%, and the product contains a small amount of impurities.
[0032] Note: When less reactants are put in, the temperature effect is not obvious, but if more reactants are put in and the exotherm is excessive, more by-products will be produced.
Embodiment 3
[0034] Add 10g 4-nitrophthalamide and 100mL DMF to a 250mL single-neck round-bottomed flask successively, place the system on a magnetic stirrer and stir, place the system in an ice bath, and wait until the system temperature drops below 5°C, Add 11.5 g of cyanuric chloride, connect the liquid sealing device, stir for 20 min, remove the ice bath, and then react at room temperature for 24 h. Then put the system in an ice bath, and when the temperature drops below 0 °C, add 15 mL of distilled water dropwise to the system, keep the temperature below 15 °C during the titration process, and pour it into 500 mL of ice-water mixture after the system is clarified, filter After washing with water and drying, 6.5 g of 4-nitrophthalonitrile was obtained with a yield of about 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com