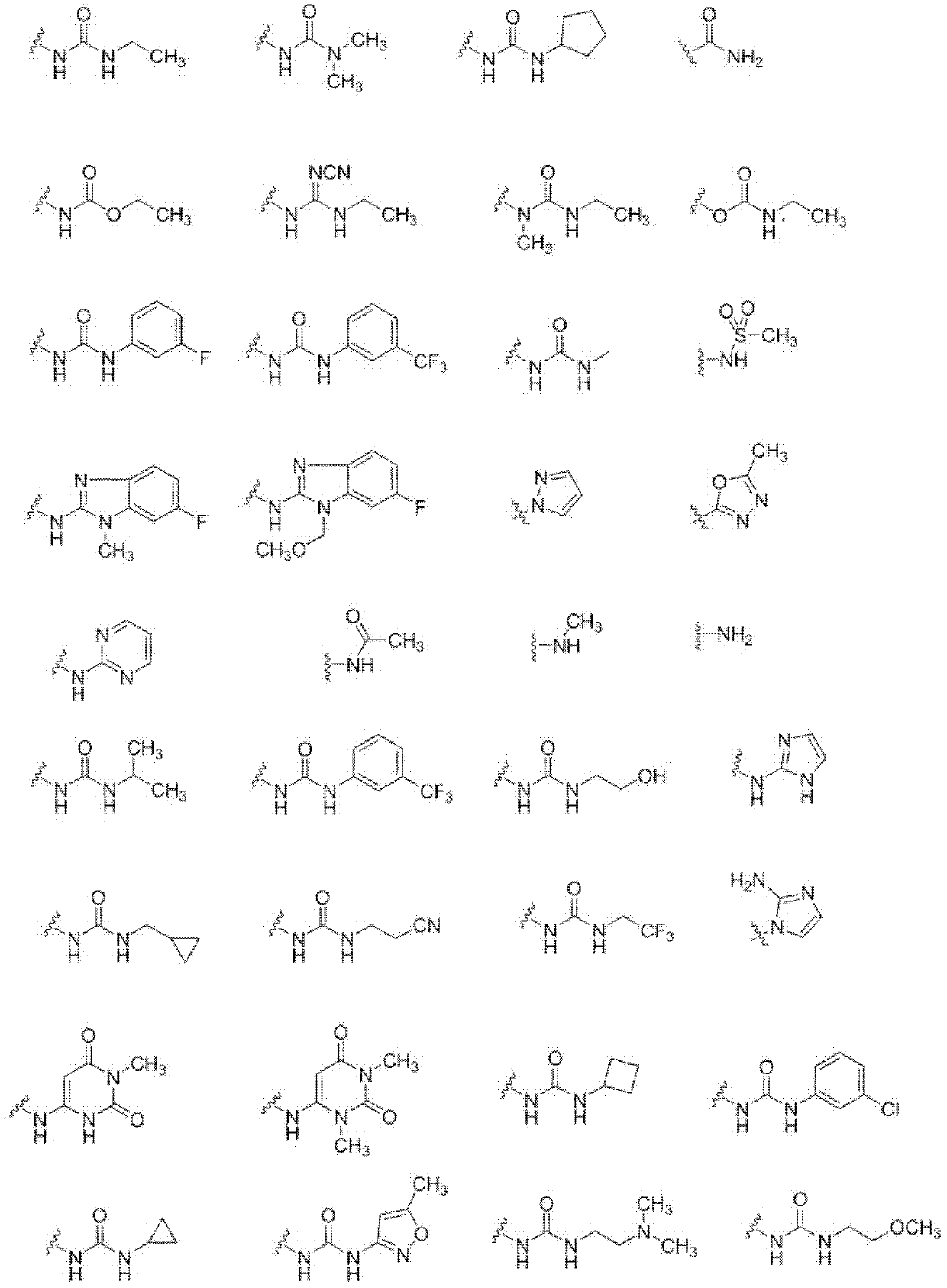
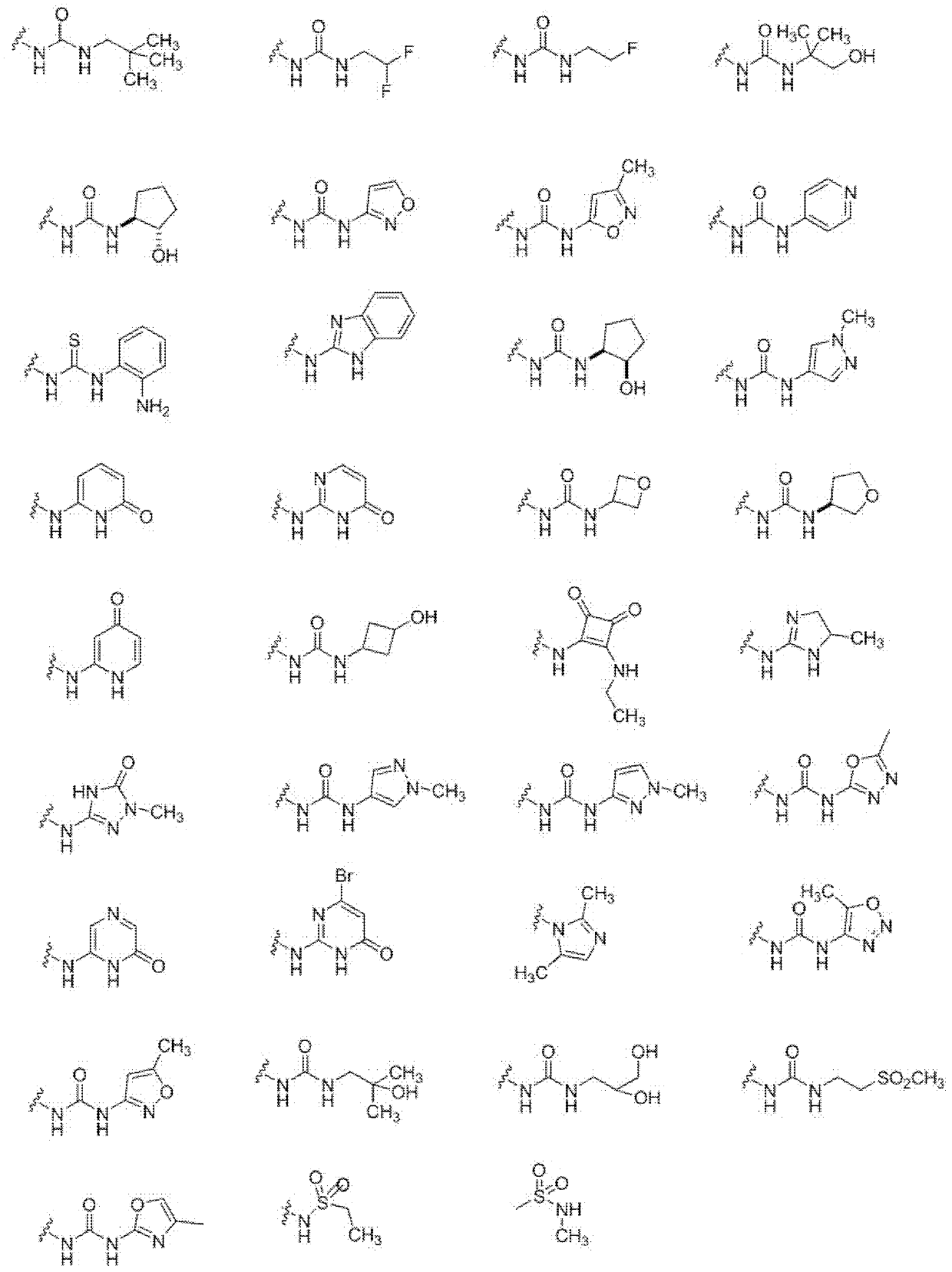
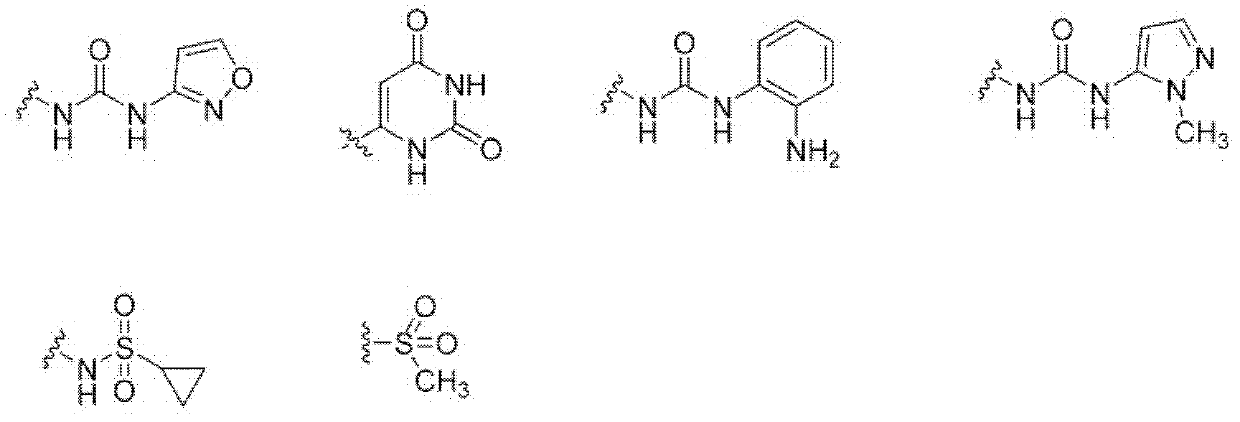
Oxo-heterocycle fused pyrimidine compounds, compositions and methods of use
A technology of compounds and heterocycles, applied in botany equipment and methods, drug combinations, applications, etc., can solve problems such as loss-of-function mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0364] Preparation of 1-ethyl-3-(4-(4-morpholino-7,8-dihydro-6H-pyrano[3,2-d]pyrimidin-2-yl)phenyl)urea (f) :
[0365]
[0366] Step 1 - Synthesis a: Dihydro-2H-pyran-3(4H)-one (9.2mL, 99.8mmol) and methyl thiocyanate (32mL, 401.0mmol) in nitromethane (75mL) at -40°C ) was added trifluoromethanesulfonic anhydride (25 mL, 148.3 mmol). The mixture was stirred at -40 °C for 6 h, then at room temperature overnight. The reaction was quenched by the slow addition of saturated aqueous sodium bicarbonate. The layers were separated and the aqueous phase was extracted with 2 x 20 mL of dichloromethane. Combined organic phases with MgSO 4 Dry, filter and concentrate. The crude material was purified by flash column chromatography (100% Hex-80% EtOAc / Hex) to give 2,4-bis(methylthio)-7,8-dihydro-6H-pyrano[3,2- d] Pyrimidine (a) (1.7 g, 7%). LC-MS: m / z=229 (M+H). 1 HNMR (400MHz, CDCl 3 ) δ 4.30-4.19 (m, 2H), 2.79 (t, J=6.6, 2H), 2.56 (s, 3H), 2.54 (s, 3H), 2.16-1.98 (m, 2H).
[...
Embodiment 2
[0373] Preparation of (S)-1-ethyl-3-(4-(4-(3-ethylmorpholino)-6,7-dihydro-5H-pyrano[2,3-d]pyrimidine-2 -yl)phenyl)urea (g):
[0374]
[0375] (S)-1-ethyl-3-(4-(4-(3-ethylmorpholino)-6,7-dihydro-5H-pyrano[2,3-d]pyrimidine-2- base)phenyl)urea (g) was prepared in a similar manner as described for Example 1, except that in step 1 tetrahydro-2H-pyran-2-one was used instead of dihydro-2H-pyran-2-one pyran-3(4H)-one and the use of (S)-3-ethylmorpholine in step 5 instead of morpholine. LC-MS: m / z=412 (M+H). 1 H NMR (500MHz, DMSO) δ8.71(s, 1H), 8.10(d, J=8.7, 2H), 7.45(d, J=8.8, 2H), 6.24(s, 1H), 4.34(s, 1H ), 4.24(s, 1H), 3.85(s, 2H), 3.77(d, J=11.3, 1H), 3.67(d, J=8.7, 1H), 3.57(t, J=11.3, 2H), 3.41 (s, 1H), 3.18-3.05(m, 2H), 2.64(s, 2H), 1.93(s, 1H), 1.77(d, J=48.0, 3H), 1.05(t, J=7.2, 3H) , 0.84 (t, J=7.5, 3H).
Embodiment 3
[0377] Preparation of 1-(4-(4-(2-oxa-5-azabicyclo[2.2.1]hept-5-yl)-6,7-dihydro-5H-pyrano[2,3- d] pyrimidin-2-yl)phenyl)-3-ethylurea (h):
[0378]
[0379]1-(4-(4-(2-oxa-5-azabicyclo[2.2.1]hept-5-yl)-6,7-dihydro-5H-pyrano[2,3-d ]pyrimidin-2-yl)phenyl)-3-ethylurea (h) was prepared in a manner similar to that described for Example 1, except that in step 1 tetrahydro-2H-pyran- 2-Kone instead of dihydro-2H-pyran-3(4H)-one and 2-oxa-5-azabicyclo[2.2.1]heptane in step 5 instead of morpholine. LC-MS: m / z=396 (M+H). 1 H NMR (400MHz, DMSO) δ8.63(s, 1H), 8.08(d, J=8.8, 2H), 7.44(d, J=8.8, 2H), 6.18(t, J=5.5, 1H), 5.01 (s, 1H), 4.61 (s, 1H), 4.34 (d, J = 11.0, 1H), 4.15 (t, J = 9.4, 1H), 3.88 (dd, J = 23.1, 7.3, 2H), 3.74 ( d, J=9.6, 1H), 3.45(d, J=9.7, 1H), 3.21-3.03(m, 3H), 2.83-2.59(m, 1H), 2.03-1.64(m, 4H), 1.06(t , J=7.2, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com