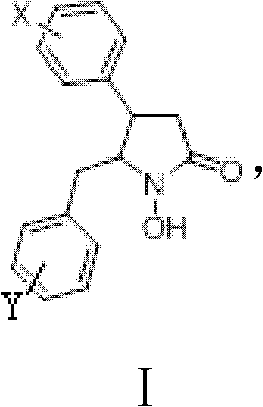
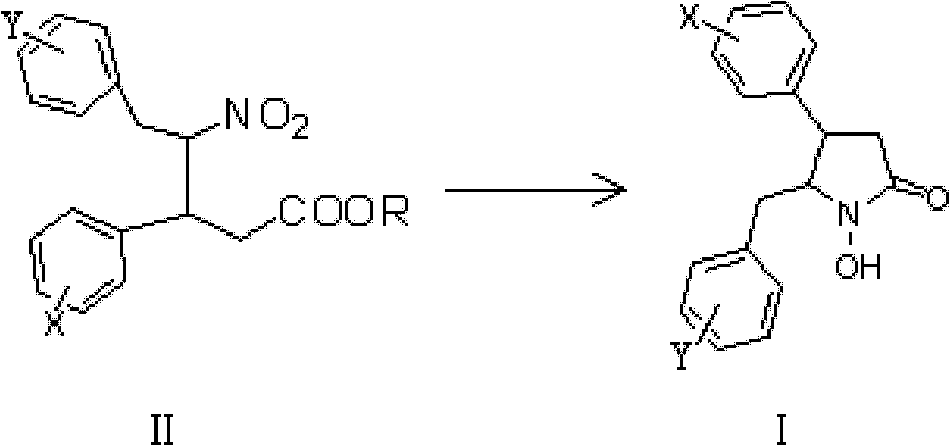
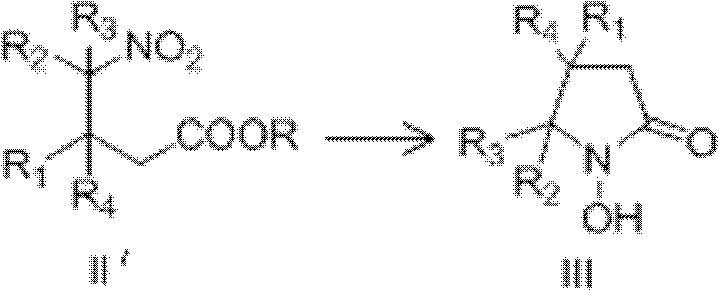
1-hydroxy-2-ketopyrrolidine compound and preparation method and application thereof
A pyrrolidone and compound technology, applied in the field of medicinal chemistry, can solve the problems that calcium antagonistic activity has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of compound DL-41
[0048] Take 57mg (0.322mmol) of nickel chloride and dissolve it in a round bottom flask with methanol, add an ice bath, and slowly add 6mg (0.161mmol) of NaBH under magnetic stirring 4 , stirred at room temperature for 30 min, and added 54 mg (0.161 mmol) of γ-8 (trans) and 312 μL (6.5 mmol) of hydrazine hydrate. Reaction at room temperature for 3h (TLC detection of reaction progress). After the reaction was completed, the methanol was distilled off under reduced pressure, the residue was dispersed with water, extracted with ethyl acetate under acidic conditions, and the organic layer was washed with anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure, and the residue is subjected to silica gel column chromatography (10:1 → 5:1 chloroform-ethanol elution) to obtain 45mg DL-41 (trans 1-hydroxyl-4-p-chlorophenyl- 5-o-chlorobenzyl-2-pyrrolidone) (yield 83.4%).
[0049]
[0050] DL-41: Light red powder, ...
Embodiment 2
[0051] Embodiment 2: the synthesis of compound DL-42
[0052] Take 53mg (0.224mmol) of nickel chloride and dissolve it in a round bottom flask with methanol, add an ice bath, and slowly add 6mg (0.149mmol) of NaBH under magnetic stirring 4 , stirred at room temperature for 30 min, and added 57 mg (0.149 mmol) of γ-8 (cis) and 289 μL (5.9 mmol) of hydrazine hydrate. Reaction at room temperature for 3h (TLC detection of reaction progress). After the reaction was completed, the methanol was distilled off under reduced pressure, the residue was dispersed with water, extracted with ethyl acetate under acidic conditions, and the organic layer was washed with anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure, and the residue is subjected to silica gel column chromatography (10:1 → 5:1 chloroform-ethanol elution) to obtain 40 mg DL-42 (cis-1-hydroxyl-4-p-chlorophenyl- 5-o-chlorobenzyl-2-pyrrolidone) (yield 70.2%).
[0053]
[0054] DL-42: Light red powder, mp6...
Embodiment 3
[0055] Embodiment 3: the synthesis of compound W-41
[0056] Take 258mg (1.088mmol) of nickel chloride and dissolve it in a round bottom flask with methanol, add an ice bath, and slowly add 24mg (0.725mmol) of NaBH under magnetic stirring 4 , stirred at room temperature for 30 min, and added 227 mg (0.725 mmol) of γ-9 (trans) and 1405 μL (29.0 mmol) of hydrazine hydrate. Reaction at room temperature for 3h (TLC detection of reaction progress). After the reaction was completed, the methanol was distilled off under reduced pressure, the residue was dispersed with water, extracted with ethyl acetate under acidic conditions, and the organic layer was washed with anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure, and the residue is subjected to silica gel column chromatography (10:1 → 5:1 chloroform-ethanol elution) to obtain 200 mg of W-41 (trans 1-hydroxyl-4-phenyl-5- benzyl-2-pyrrolidone) (yield 88.2%).
[0057]
[0058] W-41: Light red powder, mp120~124...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shrinkage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com