Separator for non-aqueous electrolyte secondary battery and non-aqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, applied in non-aqueous electrolyte storage batteries, non-aqueous electrolytes, secondary batteries, etc., can solve the problems of reduced current flow, difficulty in achieving sufficient effects, and reduced capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294]
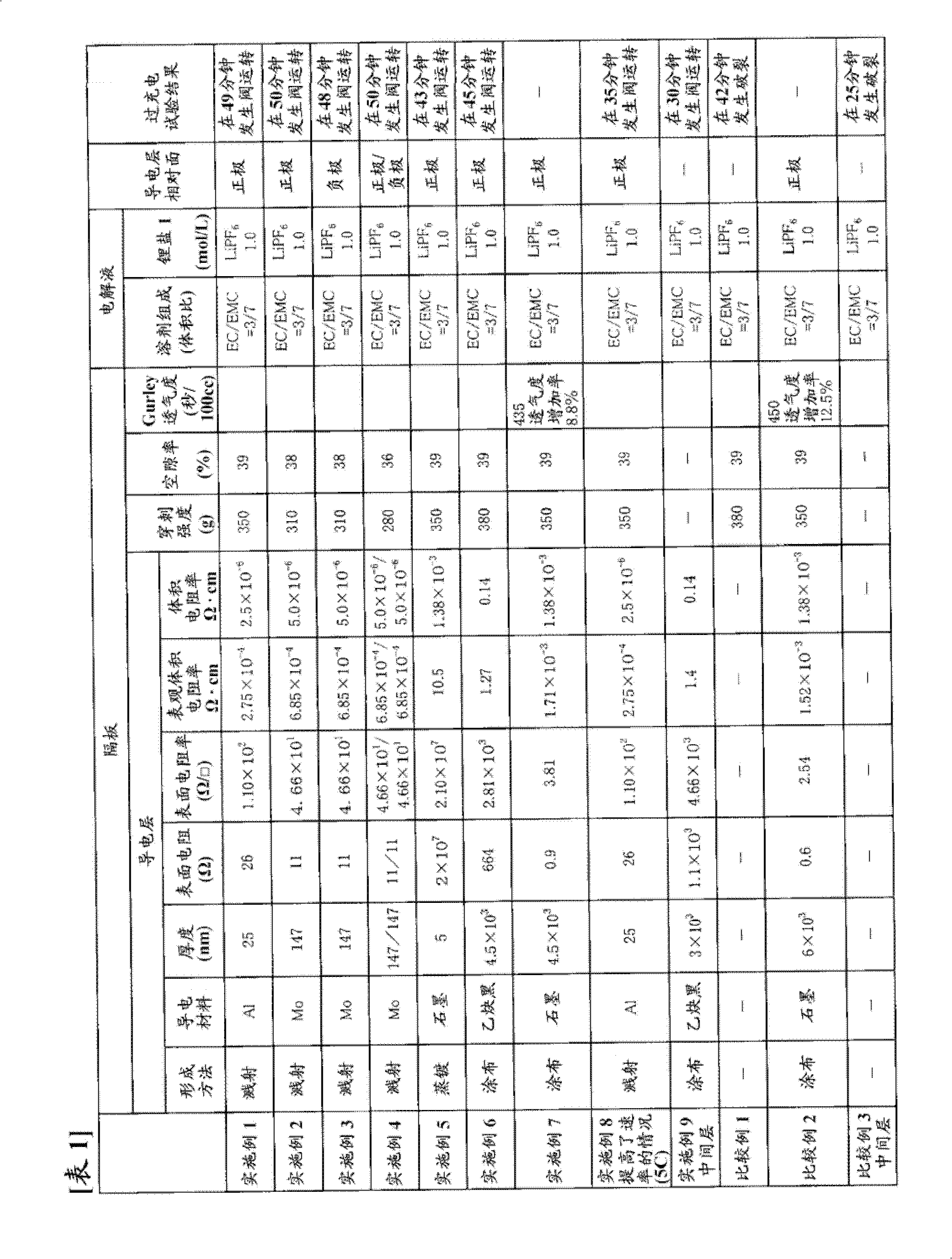
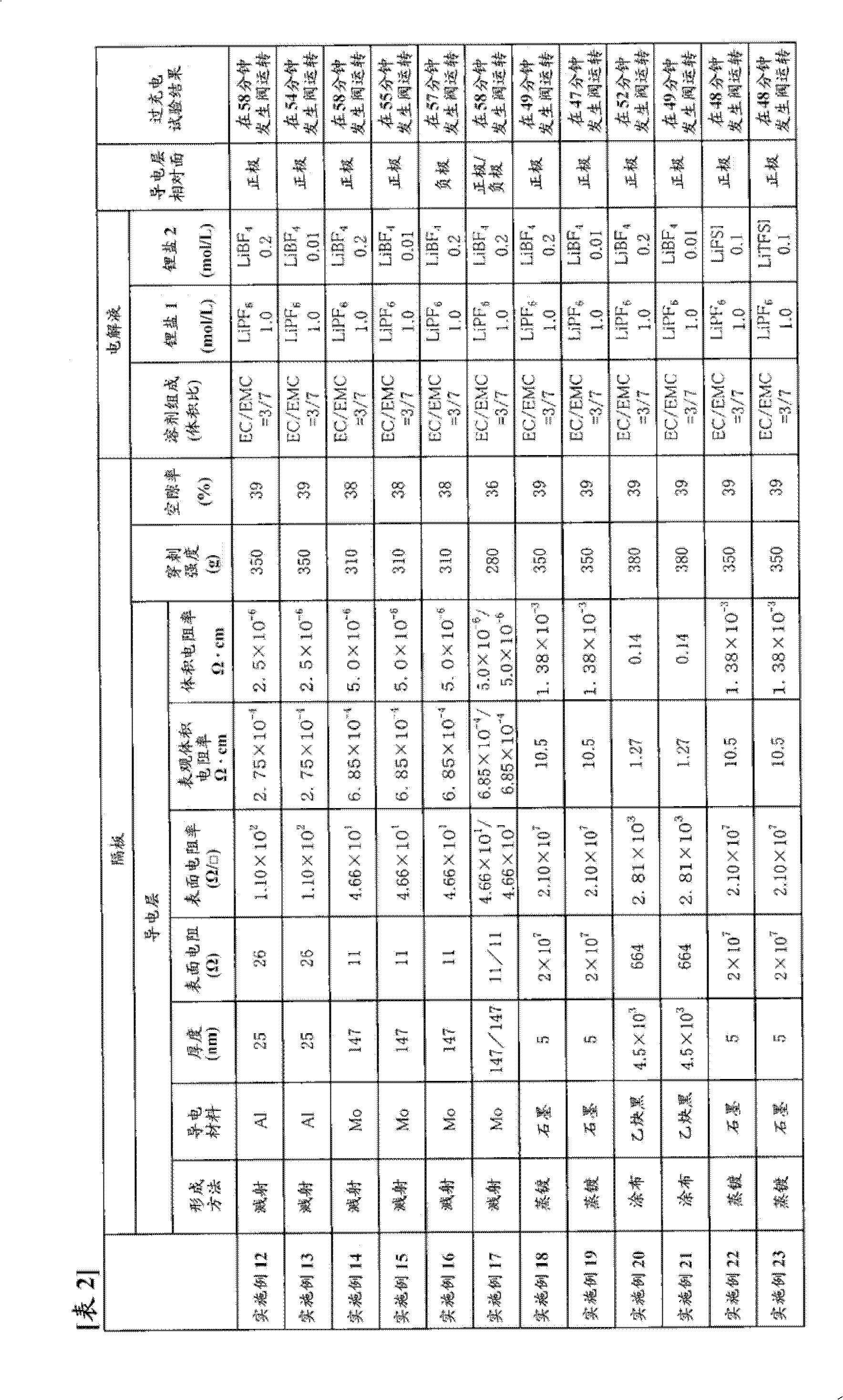
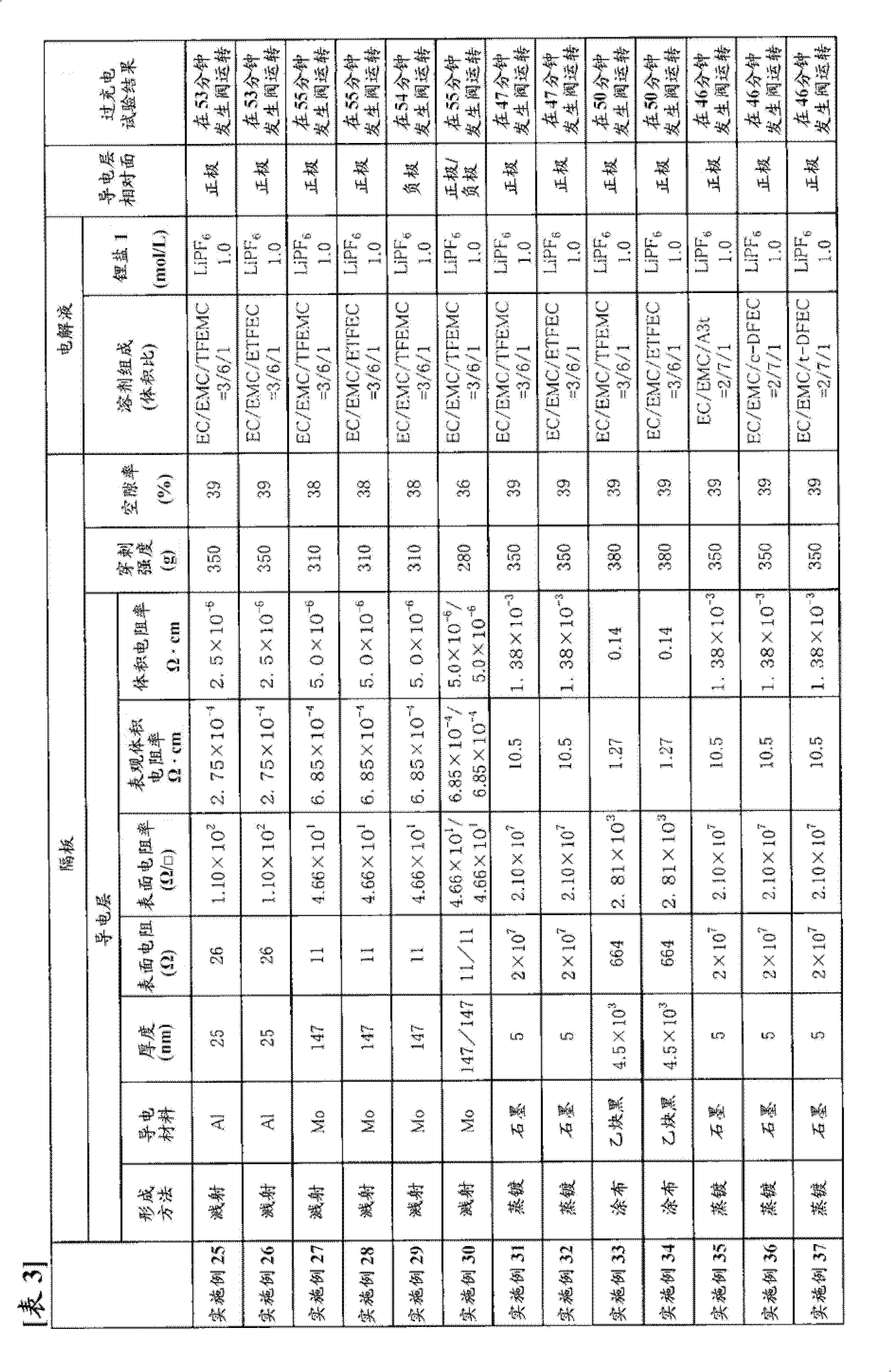
[0295] In a dry argon atmosphere, dissolve fully dried lithium hexafluorophosphate (LiPF 6 ), and make the lithium hexafluorophosphate (LiPF 6 ) to a ratio of 1.0 mol / l, thereby obtaining a non-aqueous electrolyte.
[0296]
[0297] Use LiNi 1 / 3 mn 1 / 3 co 1 / 3 o 2 As the positive active material, and to the LiNi 1 / 3 mn 1 / 3 co 1 / 3 o 2 Add 5 parts by weight of acetylene black and 5 parts by weight of polyvinylidene fluoride (manufactured by Kureha Chemical Co., Ltd., trade name "KF-1000") to 90 parts by weight, mix, and disperse the mixture in N-methyl-2-pyrrolidone In, make slurry. The resulting slurry was evenly coated on both sides of an aluminum foil with a thickness of 15 μm as a positive electrode current collector, and after drying, it was rolled to a thickness of 81 μm by a press, and cut into an active material layer having a width of 100 mm and a length of 100 mm. It had the shape of an uncoated part with a width of 30 mm, and this was used as a p...
Embodiment 2
[0309] Using the same commercially available three-layer separator with a thickness of 25 μm as used in Example 1 as a base material, one surface thereof was subjected to Mo sputtering treatment to form a conductive layer. The thickness of the Mo layer was 147 nm, and the surface resistance was 11Ω. In addition, the puncture strength of the separator was 310 g, and the porosity was 38%.
[0310] Using the obtained separator, a battery was produced in the same manner as in Example 1, and an overcharge test was performed.
[0311] The results are shown in Table 1.
Embodiment 3
[0313] A battery was produced in the same manner as in Example 1 except that the conductive layer of the separator obtained in Example 2 was opposed to the negative electrode, and an overcharge test was performed.
[0314] The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com