1,3,4 - Trisubstituted pyrrole compound and preparation method and application thereof
A compound, three-substituted technology, applied in the field of medicine, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
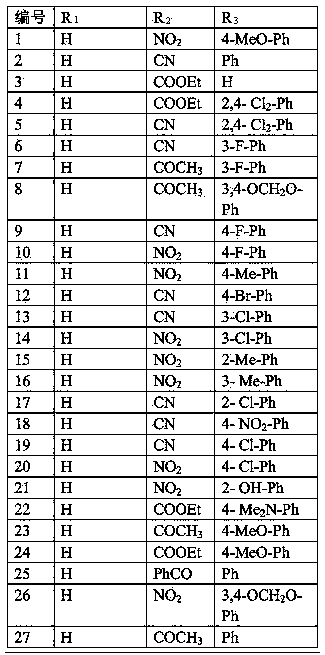
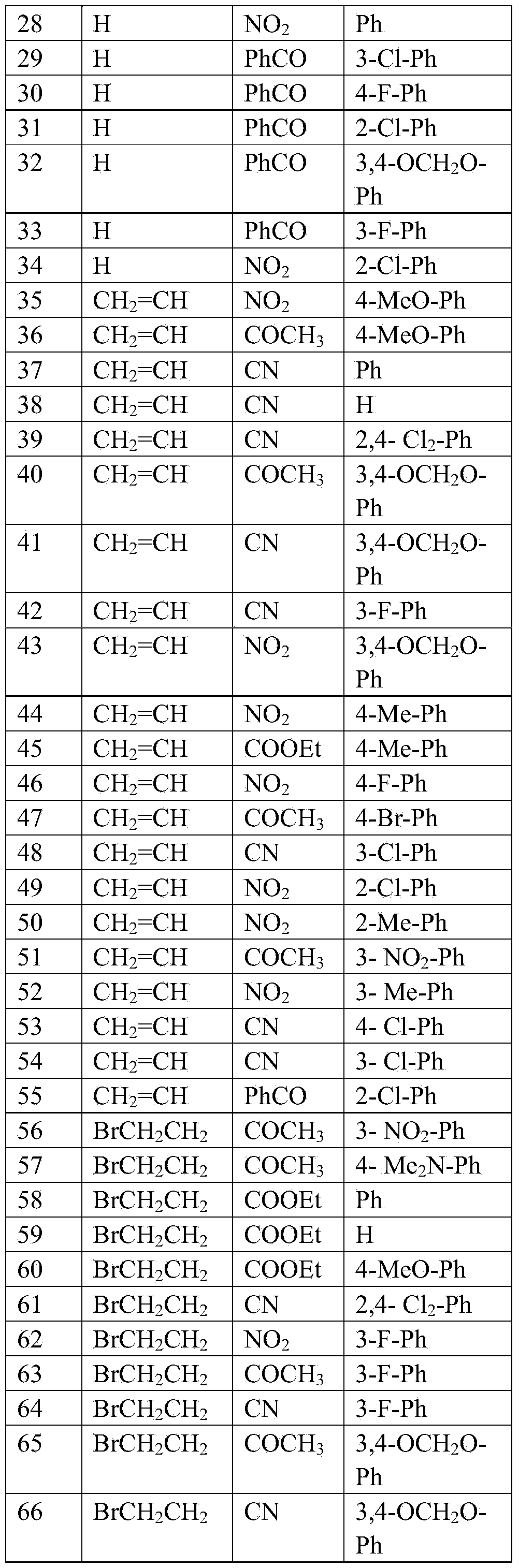
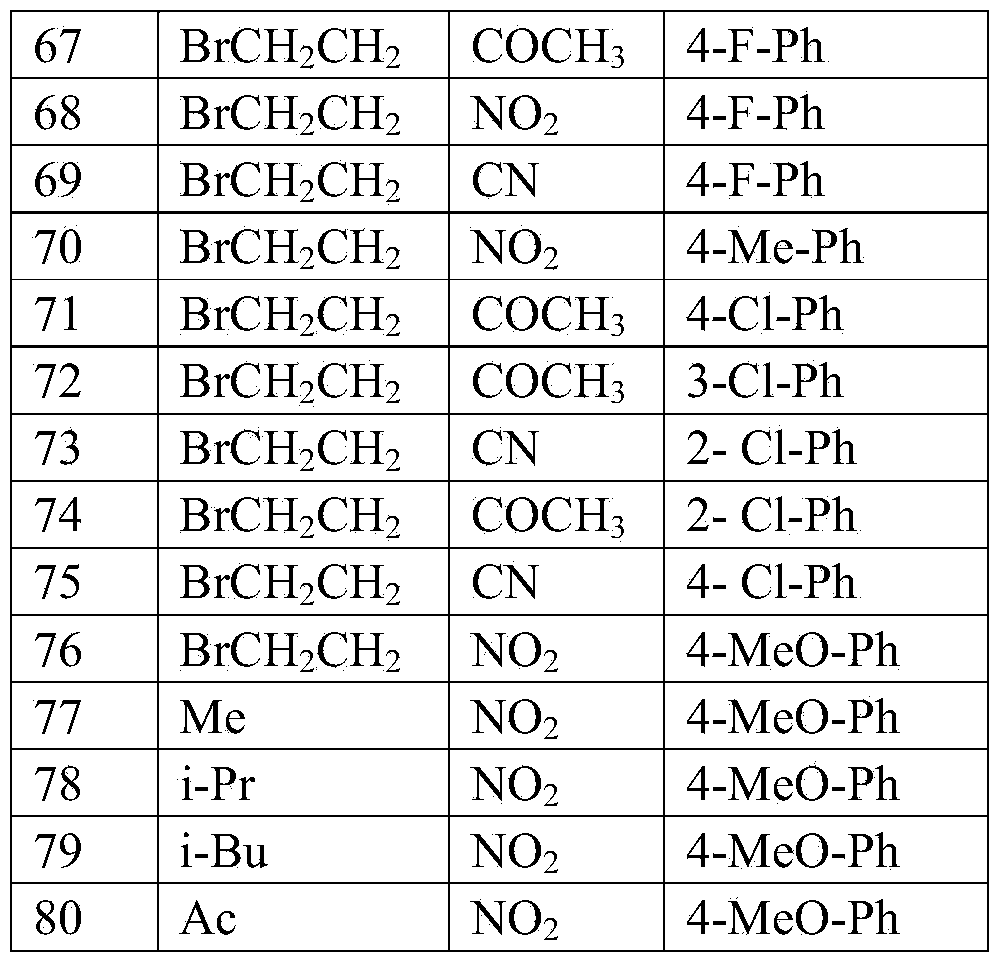
[0050] Implementation case 1, 3-(4-methoxyphenyl)-4 nitropyrrole
[0051] Under the protection of nitrogen, add 4-methoxyphenylnitroethylene (0.18g, 1mmol) and p-toluenesulfonate methyl isocyanide (0.23g, 1.2mmol) to 10mLDMSO, slowly add tert-butanol Potassium (0.15g, 1.3mmol), stirred at room temperature for 3 hours, quenched the reaction by adding saturated aqueous ammonium chloride solution, extracted three times with dichloromethane, dried over anhydrous sodium sulfate, distilled off the solvent, silica gel column chromatography (petroleum Ether: ethyl acetate=3:1), a solid (0.17g) was obtained, and the reaction yield was 79%. 1 HNMR (300MHz, CDCl 3 )δ(ppm):8.551(1H,s),7.741(1H,m),7.381(2H,d,J=4.8Hz),6.935(2H,d,J=4.2Hz),6.706(1H,m) ,3.845(3H,s).
Embodiment example 2
[0052] Implementation case 2, N-bromoethyl-3-(4-fluorophenyl)-4 cyanopyrrole
[0053] Under nitrogen protection, 3-(4-fluorophenyl)-4-cyanopyrrole (0.19g, 1mmol), 1,2-dibromoethane (0.56g, 3mmol) and potassium carbonate (1.38g , 10mmol), heated at 80°C for 8 hours, after the reaction, add 20mL of water, extract three times with dichloromethane, dry over anhydrous sodium sulfate, evaporate the solvent, silica gel column chromatography (petroleum ether: ethyl acetate = 6:1 ), a solid (67 mg) was obtained, and the reaction yield was 23%. 1 HNMR (300MHz, CDCl 3 )δ(ppm):7.933(2H,d,J=8.1Hz),7.367(2H,d,J=8.1Hz),7.249(1H,s),6.896(1H,s),4.320(2H,t, d=6.3Hz),3.622(2H,t,d=6.3Hz).
Embodiment example 3
[0054] Implementation case 3, N-methyl-3-4-methoxy-4-nitropyrrole
[0055] Under the protection of nitrogen, add 3-(4-methoxy)-4-nitropyrrole (0.218g, 1mmol), iodomethane (0.42g, 3mmol) and potassium carbonate (1.38g, 10mmol) in 15mL DMF, room temperature Stir for 5 hours. After the reaction is over, add 20 mL of water, extract three times with dichloromethane, dry over anhydrous sodium sulfate, evaporate the solvent, perform silica gel column chromatography (petroleum ether: ethyl acetate = 6:1), and obtain the product, pale Yellow solid, the reaction yield is 88%. 1 HNMR (300MHz, CDCl 3 )δ(ppm):7.555(1H,s),7.342(2H,d,J=9Hz),6.903(2H,d,J=9Hz),6.496(1H,s),3.833(3H,s),3.715 (3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com