Chiral spiro phosphoramide derivative and preparation method thereof
A technology for spirocyclic phosphonamide and derivatives, which is applied in the field of chiral spirocyclic phosphonamide derivatives and their preparation, can solve the problems of limited types of chiral phosphonamide derivatives and the like, and achieves wide range of catalytic organic reactions and good activity and the effect of enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
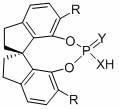
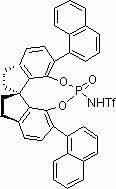
[0021] Example 1 ( S Preparation of )-O,O'-{7,7'-[6,6'-bis-(1-naphthyl)-1,1'-spirobisdihydroindane]}phosphoramide
[0022] The structural formula is as follows:
[0023]
[0024] put the compound ( S )-6,6'-bis-(1-naphthyl)-1,1'-spiroindane-7,7'-diol (10 mmol), phosphorus oxychloride and 4-N-bis The molar equivalent ratio of methylamino and triethylamine is 1:1~1.5:2:5~10 mixed in 30 ml of dichloromethane solvent, at 0-5 o After C reacted for 3 hours, 20 mmoles of trifluoromethanesulfonamide and 15 milliliters of propionitrile were added; o C reaction for 6 hours, cooling to end the reaction, then adding water and dichloromethane to extract, the organic phase was washed with dilute hydrochloric acid and saturated brine in turn, then dried with sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, and then passed through column chromatography A solid was obtained, which was dissolved in dichloromethane, washed with dilute hydrochlor...
Embodiment 2
[0025] Example 2 ( S Preparation of )-O,O'-{7,7'-[6,6'-bis-(1-naphthyl)-1,1'-spirobisdihydroindene]}thiophosphoramide
[0026] The structural formula is as follows:
[0027]
[0028] put the compound ( S )-6,6'-di-(1-naphthyl)-1,1'-spiroindane-7,7'-diol (10 mmol), phosphorus tribromide and triethylamine in moles The equivalent ratio is 1:1.2:10 mixed in 40 ml of toluene solvent, at 35 o After C reacted for 3 hours, 30 mmol of trifluoromethanesulfonamide was added; o C reaction for 6 hours, then add 20 mmol of sulfur powder, react at 110 degrees for 24 hours; then cool to end the reaction, add water and dichloromethane for extraction, the organic phase is washed with dilute hydrochloric acid and saturated saline successively, and then washed with sodium sulfate Drying, filtration, the filtrate was concentrated to dryness under reduced pressure, and then obtained a solid by column chromatography, the gained solid was dissolved in dichloromethane, washed with dilute hydr...
Embodiment 3
[0029] Embodiment 3 ( S )-O, O'-{7,7'-[6,6'-two-(1-naphthyl)-1,1'-spirobisdihydroindane]} phosphorothioate preparation, the structural formula is as follows:
[0030]
[0031] put the compound ( S )-6,6'-di-(1-naphthyl)-1,1'-spiro indane-7,7'-diphenol (10 mmol), phosphorus pentasulfide in a molar equivalent ratio of 1:0.5 In 40 ml of xylene solvent, at 120 o C reacted for 5 hours, cooled to end the reaction, then added water and dichloromethane to extract, the organic phase was washed with dilute hydrochloric acid and saturated brine in turn, then dried with sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, and then passed through the column layer The obtained solid was dissolved in dichloromethane, washed with dilute hydrochloric acid, then the organic phase was concentrated under reduced pressure, and vacuum-dried to obtain ( S )-O,O'-{7,7'-[6,6'-di-(1-naphthyl)-1,1'-spirobisdihydroindene]}thiophosphoric acid, code name is Cata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com