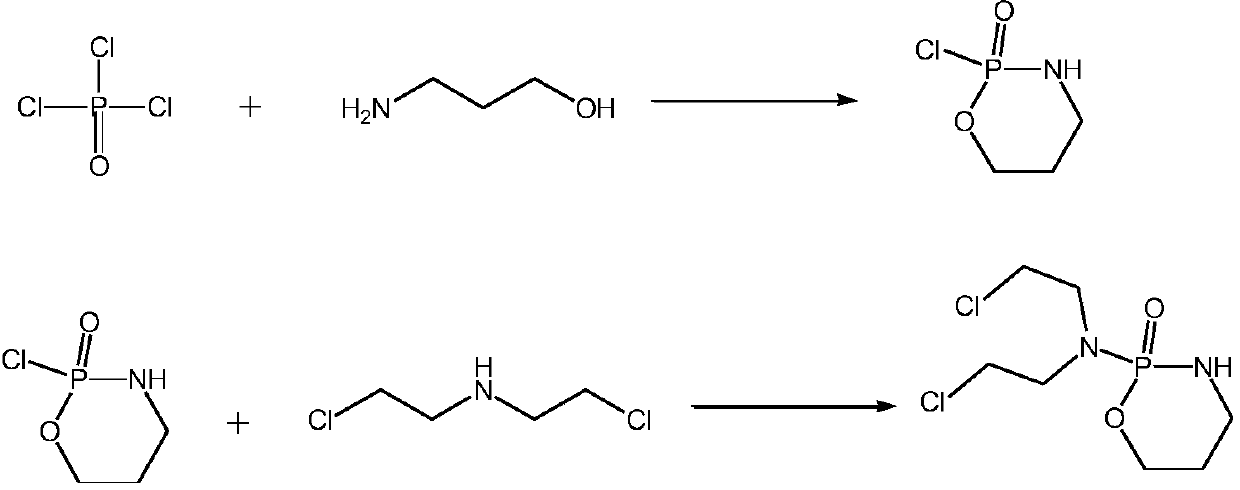
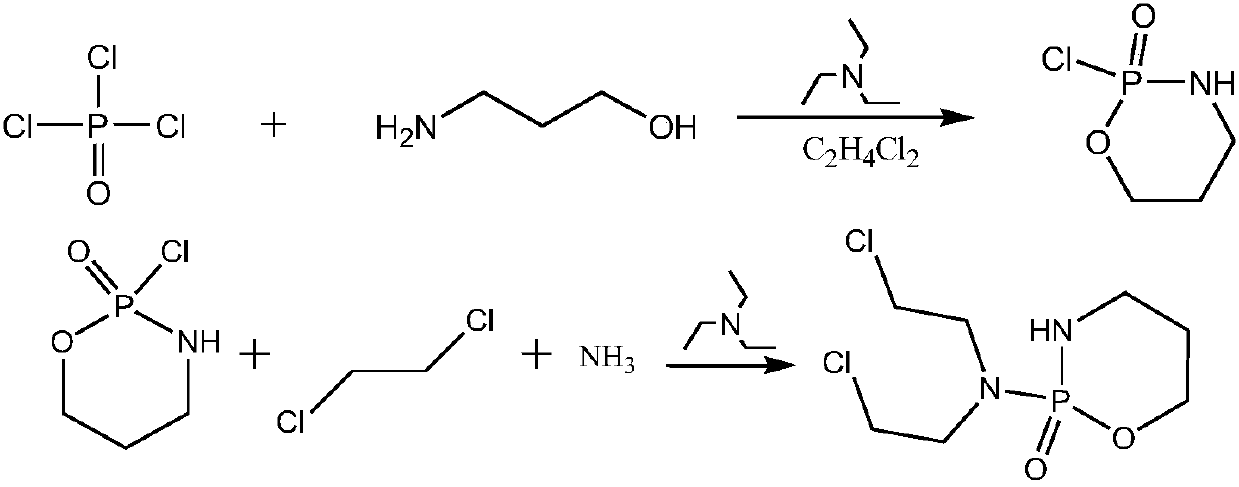
Cyclophosphamide synthetic method
A synthetic method and technology of cyclophosphamide, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield and many side reactions, and achieve the effects of high yield, simple steps and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Add 500ml of dichloroethane into a 1000ml reaction flask, slowly add 60g of phosphorus oxychloride, cool down to -5°C, start to add dropwise a mixed solvent of 30g of 3-aminopropanol and 60g of triethylamine, and add dropwise After completing the reaction for 18 hours, a 2-chloro-2-oxo-[1.3.2]oxazaphosphorinane solution was obtained, and the solution was transferred into a pressure reaction flask, 60 g of triethylamine was added, and the temperature was controlled at 20° C. Pass through ammonia gas, keep the pressure not lower than 0.05mpa and react for 2 hours. After the reaction is completed, transfer the reaction solution to a 1000ml reaction bottle, add 200ml of ice-water mixture, stir for 30 minutes, separate the organic phase, and add 200ml of 10% hydrochloric acid solution Wash, separate the organic phase, control the temperature of the water bath to 50°C and concentrate the organic phase to dryness under reduced pressure, add 80ml of purified water, he...
Embodiment 2
[0031] Example 2. Add 500ml of dichloroethane into a 1000ml reaction flask, slowly add 70g of phosphorus oxychloride, cool down to -5°C, start to dropwise add a mixed solvent of 35g of 3-aminopropanol and 69g of triethylamine, and add dropwise After completing the reaction for 18 hours, a 2-chloro-2-oxo-[1.3.2]oxazaphosphorinane solution was obtained, and the solution was transferred to a pressure reaction flask, 69 g of triethylamine was added, and the temperature was controlled at 21° C. Pass through ammonia gas, keep the pressure not lower than 0.05mpa and react for 2 hours. After the reaction is completed, transfer the reaction solution to a 1000ml reaction bottle, add 200ml of ice-water mixture, stir for 30 minutes, separate the organic phase, and add 200ml of 10% hydrochloric acid solution Wash, separate the organic phase, control the temperature of the water bath to 50°C and concentrate the organic phase to dryness under reduced pressure, add 80ml of purified water, heat...
Embodiment 3
[0032] Example 3. Add 500ml of dichloroethane into a 1000ml reaction flask, slowly add 66g of phosphorus oxychloride, cool down to -5°C, start to dropwise add a mixed solvent of 33g of 3-aminopropanol and 60g of triethylamine, dropwise add After completing the reaction for 18 hours, a 2-chloro-2-oxo-[1.3.2]oxazaphosphorinane solution was obtained, and the solution was transferred into a pressure reaction flask, 60 g of triethylamine was added, and the temperature was controlled at 20° C. Pass through ammonia gas, keep the pressure not lower than 0.05mpa and react for 2.3 hours. After the reaction is completed, transfer the reaction solution to a 1000ml reaction bottle, add 200ml of ice-water mixture, stir for 30 minutes, separate the organic phase, and add 200ml of 10% hydrochloric acid solution Wash, separate the organic phase, control the temperature of the water bath to 50°C and concentrate the organic phase to dryness under reduced pressure, add 80ml of purified water, heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com